Synthesis of alpha-tocopherolquinone derivatives, and methods of using the same
A technology for tocopherol quinone and tocopherol, applied in the field of synthesizing the compound of formula I, can solve the problem of high concentration of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
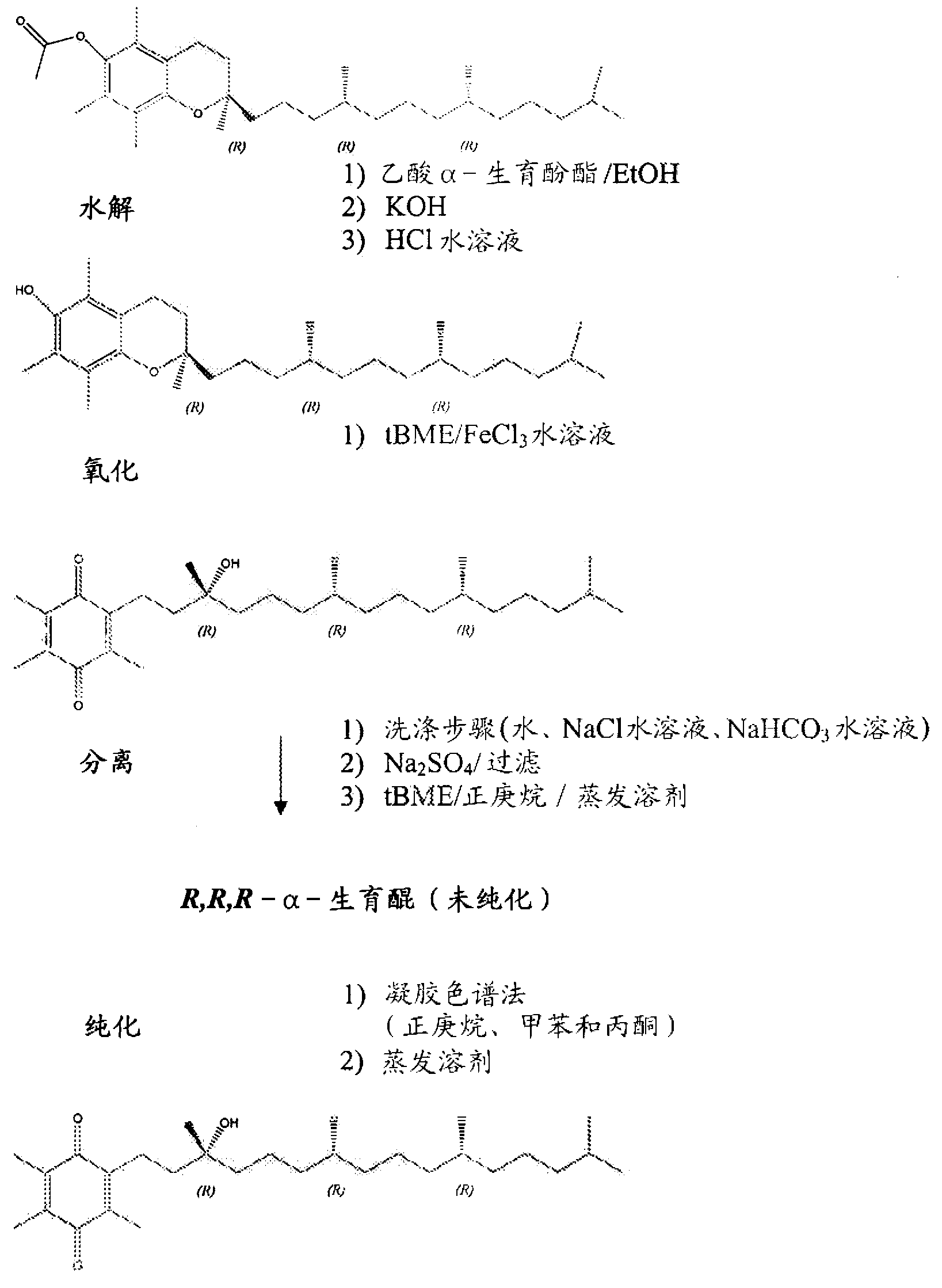
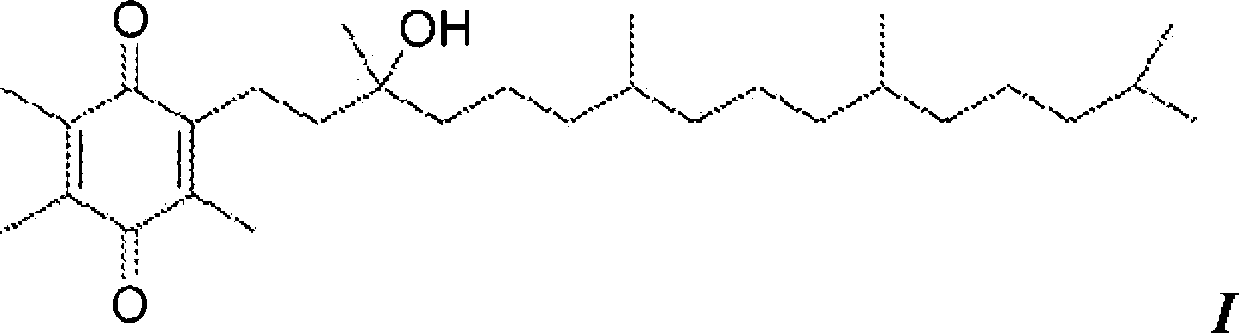
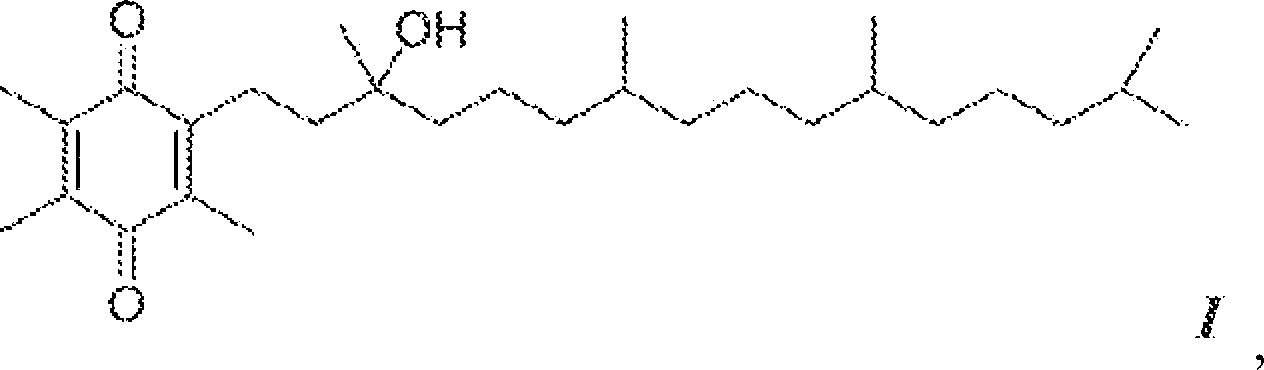
[0092] By charging an appropriate amount of R,R,R-α-tocopheryl acetate (7.0 kg) into a 72 L reaction vessel, α-tocopheryl acetate (Vita-Solar Biotechnology Co., China) was hydrolyzed to obtain the unisolated Intermediate of α-tocopherol. Dissolve α-tocopheryl acetate in ethanol (200 Proof denaturation with 0.05% toluene). The reaction vessel was cooled to 10-12°C and potassium hydroxide pellets (≥85% A.C.S. grade) were added to the flask while maintaining the temperature at 10-15°C. The mixture was stirred for 1 more hour. After at least 1 hour, the completion of the reaction was checked by sampling the batch and analyzing it by HPLC analysis.
[0093] The reaction is complete when the alpha-tocopheryl acetate is less than 0.5 area percent at 205 nm. The hydrolysis reaction product was neutralized with hydrochloric acid solution (34%-39% aqueous solution) in a reaction vessel to obtain α-tocopherol. The alpha-tocopherol solution was transferred to a 100 L separatory funnel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com