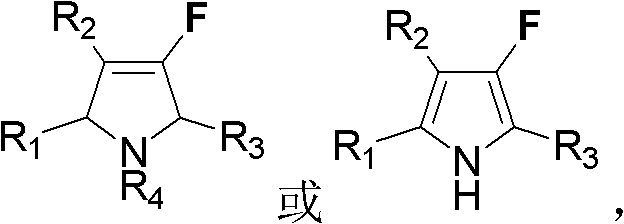
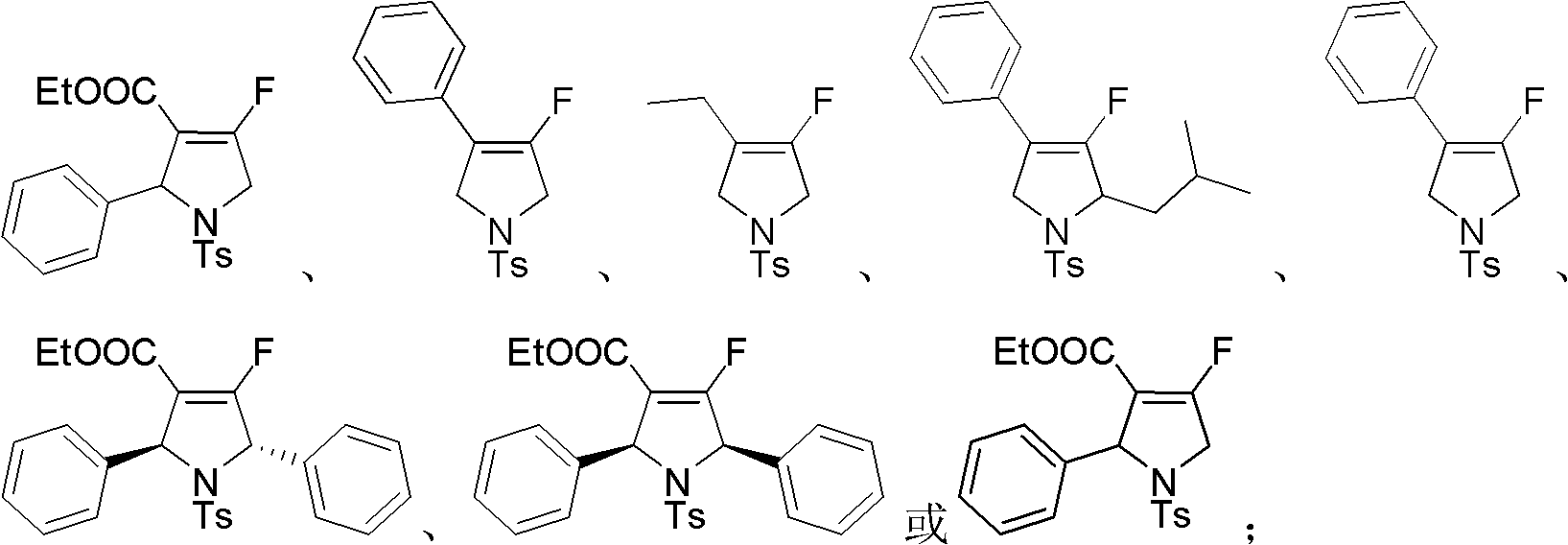
Fluoro dihydropyrrole or fluoro pyrrole
A technology of fluorinated dihydropyrrole and substituted dihydropyrrole, which is applied in the field of fluorinated dihydropyrrole or fluoropyrrole and its preparation from allenes, and can solve problems such as cumbersome routes, poor substrate compatibility, and harsh conditions , to achieve the effect of simple steps, high yield and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0036] Example 1: Synthesis
[0037] 3.4mg (0.02mmol) of AgNO 3 , 27.6 mg (0.4 mmol) of K 2 CO 3 , 47.5mg (0.3mmol) of NFSI, 37.1mg (0.1mmol) of allene Add it into the reaction tube, replace the nitrogen, add 1.5ml of anhydrous diethyl ether, and stir at 30°C for 8 hours. Then filter, wash the solid with ethyl acetate, combine the filtrate, concentrate, column chromatography, and obtain 33.5mg product with ethyl acetate and petroleum ether gradient washing method The yield was 86%.
[0038] 1 H NMR (400MHz, CDCl3) δ7.40(d, J=8.0Hz, 2H), 7.29-7.22(m, 5H), 7.16(d, J=8.0Hz, 2H), 5.70(dt, J=5.6, 2.0Hz, 1H), 4.48(dt, J=15.6, 2.4Hz, 1H), 4.36(dt, J=15.6, 5.2Hz, 1H), 4.12-3.95(m, 2H), 2.38(s, 3H), 1.10(t, J=7.2Hz, 3H); 13 C NMR (100MHz, CDCl 3 )δ160.5 (d, J=298.2Hz), 160.0 (d, J=3.7Hz), 143.7, 138.9 (d, J=1.5Hz), 134.9, 129.6, 128.3, 128.3, 127.7, 127.1, 110.3, 66.7 (d, J=2.3Hz), 60.8, 50.5 (d, J=28.2Hz), 21.4, 13.8; 19 F NMR (376MHz, CDCl 3 )δ-107.4 (dt, J=5.2, 2.0Hz...
example 2
[0039] Example 2: Synthesis
[0040] 3.4mg (0.02mmol) of AgNO 3 , 27.6 mg (0.4 mmol) of K 2 CO 3 , 47.5mg (0.3mmol) of NFSI, 44.7mg (0.1mmol) of allene Add it into the reaction tube, add 1.5ml of anhydrous diethyl ether after pumping nitrogen, and stir at 30°C for 10 hours. Then filter, wash the solid with ethyl acetate, combine the filtrate, concentrate, column chromatography, and obtain 19.3mg product with ethyl acetate and petroleum ether gradient washing method and 19.3 mg of product The overall yield was 83%.
[0041] trans-4i
[0042] trans-4i
[0043] 1 H NMR (400MHz, CDCl3) δ7.45-7.35(m, 4H), 7.35-7.27(m, 6H), 7.12(d, J=8.4Hz, 2H), 6.98(d, J=8.4Hz, 2H) , 5.91(dd, J=6.4, 1.6Hz, 1H), 5.74(s, 1H), 4.15-3.95(m, 2H), 2.30(s, 3H), 1.07(t, J=7.6Hz, 3H); 13 C NMR (100MHz, CDCl 3 )δ161.1 (d, J=298.9Hz), 160.3, 143.5, 128.8, 135.8, 135.3, 129.2, 128.9, 128.6 (d, J=6.7Hz), 128.4, 128.3, 128.0, 127.3, 125.5 (d, J =6.7Hz), 108.8, 65.7, 65.6 (d, J=24.5Hz), 60.9, 21....
example 3
[0047] Example 3: Synthesis
[0048] 3.4mg (0.02mmol) of AgNO 3 , 27.6 mg (0.4 mmol) of K 2 CO 3 , 47.5mg (0.3mmol) of NFSI, 29.9mg (0.1mmol) of allene Put it into the reaction tube, add 1.5ml of anhydrous diethyl ether after replacing the nitrogen, and stir at 30°C for 24 hours. Then filter, wash the solid with ethyl acetate, combine the filtrate, concentrate, column chromatography, and obtain 20.6mg product with ethyl acetate and petroleum ether gradient washing method The yield is 65%.
[0049] 1 H NMR (400MHz, CDCl3) δ7.77 (d, J = 8.4Hz, 2H), 7.37-7.32 (m, 6H), 7.31-7.24 (m, 1H), 4.43 (dt, J = 4.4, 4.0Hz, 2H), 4.29(dt, J=4.4, 4.0Hz, 2H), 2.42(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ 148.9 (d, J = 278.9Hz), 144.0, 133.3, 130.0, 129.6 (d, J = 4.7Hz), 128.6, 128.1 (d, J = 2.0Hz), 127.5, 126.3 (d, J = 6.3 Hz), 110.1(d, J=2.8Hz), 52.5(d, J=5.1Hz), 50.8(d, J=31.6Hz), 21.5; 19 F NMR (376MHz, CDCl 3 )δ-126.0 (tt, J=4.8, 4.1Hz); HRMS: calculated value of m / z (EI) [M] + :...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com