Skin hyperpigmentation acyl glutathione treatments
A technology of acyl glutathione and skin whitening, which is applied in skin diseases, skin care preparations, tripeptide components, etc., and can solve the problems of cysteine utilization rate limitation and glutathione synthesis limitation
Inactive Publication Date: 2012-12-26
N V PERRICONE
View PDF14 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
They themselves limit further glutathione synthesis due to elevated glutathione levels; additionally, cysteine availability is often rate-limiting
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
[0069] Formulation: An oil-in-water emulsion formulation was prepared by combining the following ingredients using conventional mixing techniques.
[0070] raw material
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
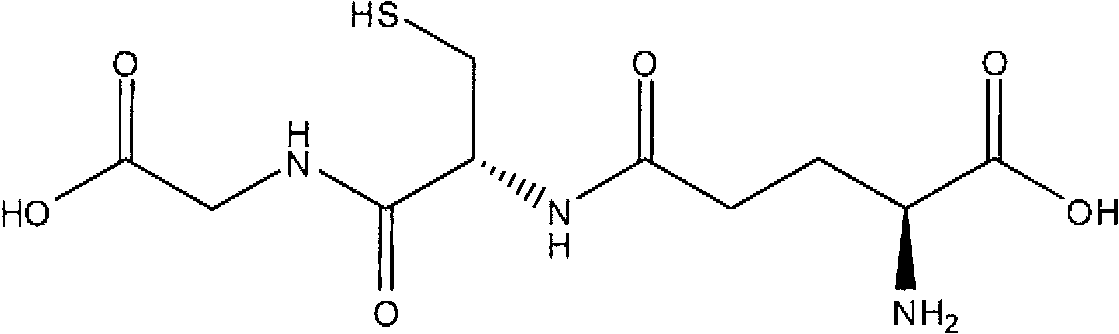
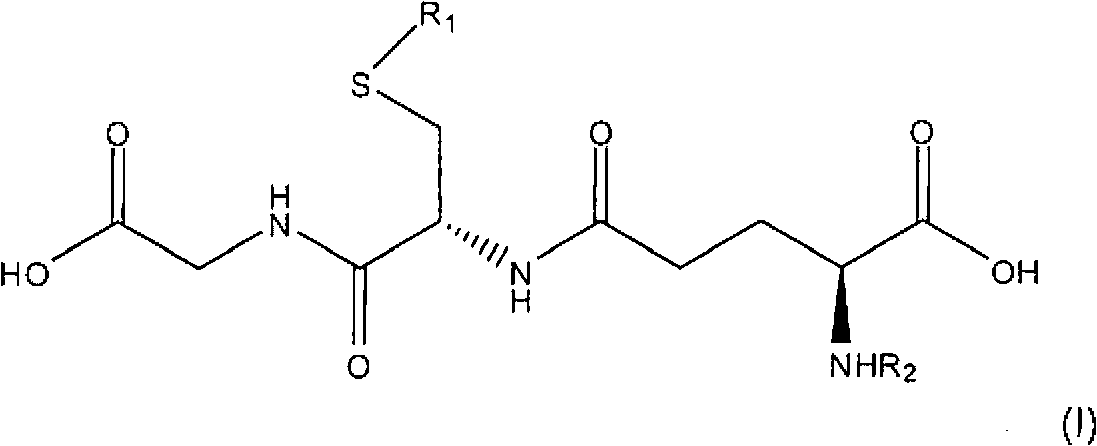
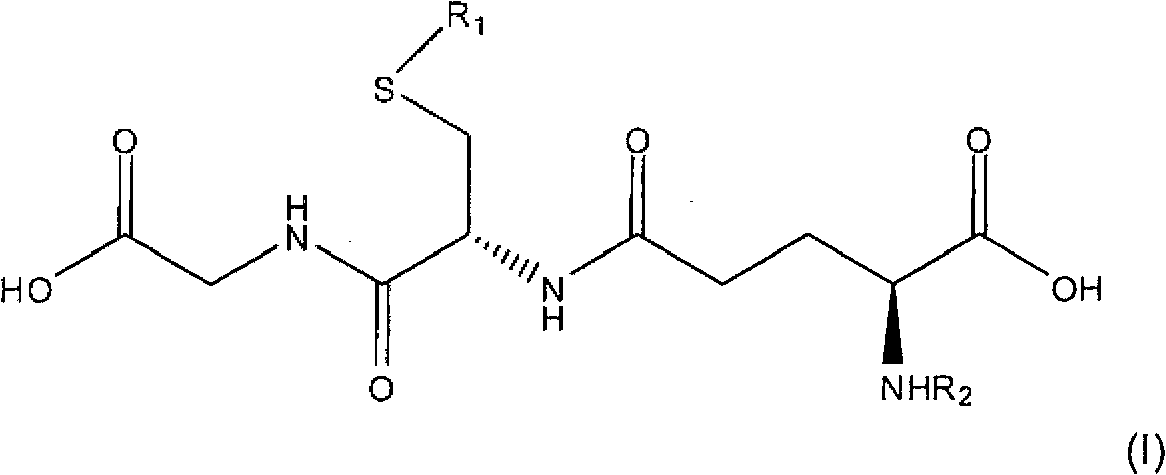
Topical compositions for treatment of hyperpigmentation comprise an effective amount of S-acyl glutathione derivative and a carrier. Methods for treating hyperpigmentation, such as melasma, postinflammatory hyperpigmentation and lentigines, skin lightening and skin whitening comprise applying a composition containing S-acyl glutathione derivative in a dermatologically acceptable carrier to skin tissue. The acyl group is a saturated or unsaturated aliphatic C12-C24 group, preferably a C16-C24 group, most preferably a C16 group. In particularly preferred embodiments, the S-acyl glutathione derivative is S-palmitoyl glutathione.
Description
field of invention [0001] The present invention relates to topical compositions comprising glutathione derivatives. More specifically, the present invention relates to topical compositions comprising glutathione acyl derivatives for skin lightening, skin lightening and treatment of skin hyperpigmentation. Background of the invention [0002] Reduced glutathione, commonly referred to as glutathione or GSH, is a relatively small molecule found in plants and animals with the following formula: [0003] [0004] Glutathione is an orthomolecule in water. It is the smallest intracellular thiol molecule. Due to its remarkable electron-donating ability, it is an effective reducing compound. Glutathione is a potent antioxidant and enzyme cofactor that plays an important role in regulating cellular activity. [0005] Glutathione is a chain tripeptide of L-glutamine, L-cysteine and glycine. Academically, N-L-γ-glutamyl-cysteinylglycine or L-glutathione, the molecule has a sul...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61Q19/02
CPCA61K8/676A61K8/678A61K8/64A61K8/4986A61K8/365A61Q19/08A61Q19/02A61P17/00A61K38/06
Inventor N.V.佩里科恩
Owner N V PERRICONE
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com