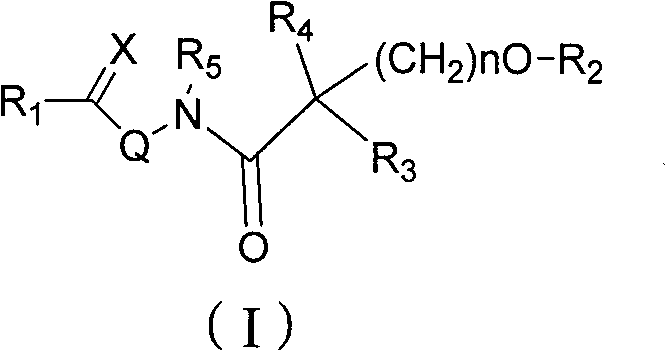
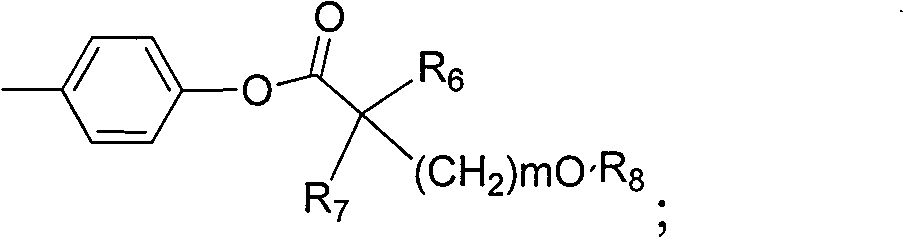
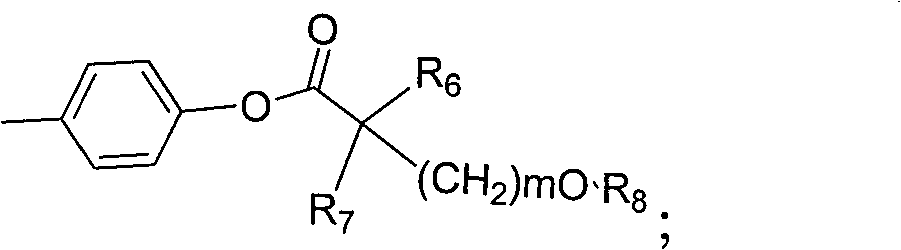
Amide compound and preparation method as well as application thereof
一种化合物、选自的技术,应用在有机化合物的制备、亚氨基化合物制备、羧酸酰胺制备等方向,能够解决肌酸激酶升高、病人肝功能损害、转氨酶升高等问题,达到降低胆固醇和低密度脂蛋白的、良好胆固醇和低密度脂蛋白的、毒性低的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Embodiment 1: the preparation of compound 01
[0092] 2-(4-(2-(4-chlorobenzamido)ethyl)phenoxy)-2-methylpropionic acid 1g, dicyclohexylcarbodiimide 0.57g, 2-amino-1- 0.5 g of (4-hydroxyphenyl) ethyl ketone and 60 ml of dichloromethane were placed in a 100 ml single-necked bottle, reacted at room temperature for 4 hours, the solvent was evaporated to dryness, and 820 mg of the target compound was obtained by silica gel column chromatography. MS(ESI): 495(M+H + ). 1 H-NMR: 7.720-7.698 (2H, d), 7.629 (1H, s), 7.605-7.584 (2H, d), 7.281 (1H, s), 7.232-7.210 (2H, d), 7.158 (1H, s ), 7.011-6.99 (2H, d), 6.810-6.751 (4H, m), 4.559-4.547 (2H, s), 3.484-3.473 (2H, m), 2.764-2.729 (2H, m), 1.381 (6H , s)
Embodiment 2
[0093] Embodiment 2: the preparation of compound 02
[0094] 2-(4-(2-(4-chlorobenzamido)ethyl)phenoxy)-2-methylpropionic acid 2g, dicyclohexylcarbodiimide 1.2g, 2-amino-1- 0.5 g of (4-hydroxyphenyl)ethanone and 60 ml of dichloromethane were placed in a 100 ml single-necked bottle, reacted at room temperature for 4 hours, evaporated to dryness, and 1.3 g of the target compound was obtained by silica gel column chromatography. MS (ESI): 838 (M+H + ).
Embodiment 3
[0095] Embodiment 3: the preparation of compound 03
[0096] 2-methyl-2-(4-(4-chlorobenzoyl)phenoxy)propionic acid 1g, dicyclohexylcarbodiimide 0.57g, 2-amino-1-(4-hydroxyphenyl) 0.5 g of ethyl ketone and 60 ml of dichloromethane were placed in a 100 ml single-necked bottle, reacted at room temperature for 4 hours, the solvent was evaporated to dryness, and 820 mg of the target compound was obtained by silica gel column chromatography. MS(ESI): 452(M+H + ). 1 H-NMR: 7.8-7.85 (2H, d), 7.7-7.8 (4H, m), 7.6 (1H, m), 7.42-7.53 (2H, d), 7.04-7.13 (2H, d), 6.9-7.1 (2H, d), 6.84 (1H, s), 4.7 (2H, d), 1.65 (6H, s)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com