Liquid formulation of follicle stimulating hormone
A follicle-stimulating hormone and preparation technology, which is applied in the directions of medical preparations without active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0071] Production of Recombinant Human FSH by Recombinant DNA Technology
[0072] Recombinant human FSH is produced in transfected CHO host cells by standard methods.
example 2
[0074] Preparation of hFSH aqueous formulations using trehalose and mannitol
[0075] Phosphate buffer at pH 7.0 was prepared using a formulation buffer comprising monobasic sodium phosphate monohydrate and disodium hydrogen phosphate dehydrate. Mannitol, trehalose and sodium chloride were added to the phosphate buffer as stress regulators and stabilizers. L-methionine as an antioxidant and polysorbate 20 as a stabilizer were added to the solution to form a formulation buffer, pH was adjusted with phosphoric acid solution or sodium hydroxide solution to obtain a pH of 7.0 ± 0.2 . The volume was brought to 100% with WFI and the solution was stirred well. The required amount of formulation buffer is mixed with phenol to form a homogeneous mixture. The calculated amount of rHu FSH drug substance was added with continuous stirring until a homogeneous mixture was fully formed.
[0076] Table 1: Formulations of hFSH
[0077] serial number
example 3
[0078] Example 3: Stability testing of hFSH aqueous formulations
[0079] Biological tests were carried out in compliance with European Pharmacopoeia specifications.
[0080] Check protein concentration by SE-HPLC
[0081] The formulation of Example 1 was evaluated for protein content of FSH using size exclusion HPLC.
[0082] Protein content was measured at time zero and after storage of the formulations at 5±3°C, 25±2°C for 1, 2, 3 and 6 months. The results are listed in Table 1 as micrograms of FSH per gram of solvent.
[0083] Reverse Phase HPLC Oxidation Analysis
[0084] The protein content of FSH was assessed on the aqueous formulation of Example 1 using a reverse phase HPLC method.
[0085] Purity was measured at time zero and after storage of the formulations at 5±3°C, 25±2°C for 1, 2, 3 and 6 months. The results are listed in Table 2.
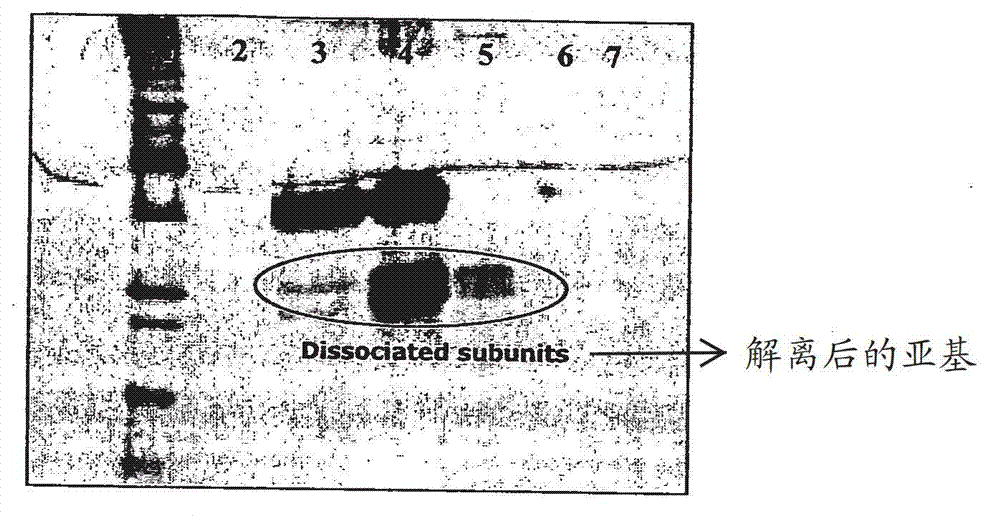
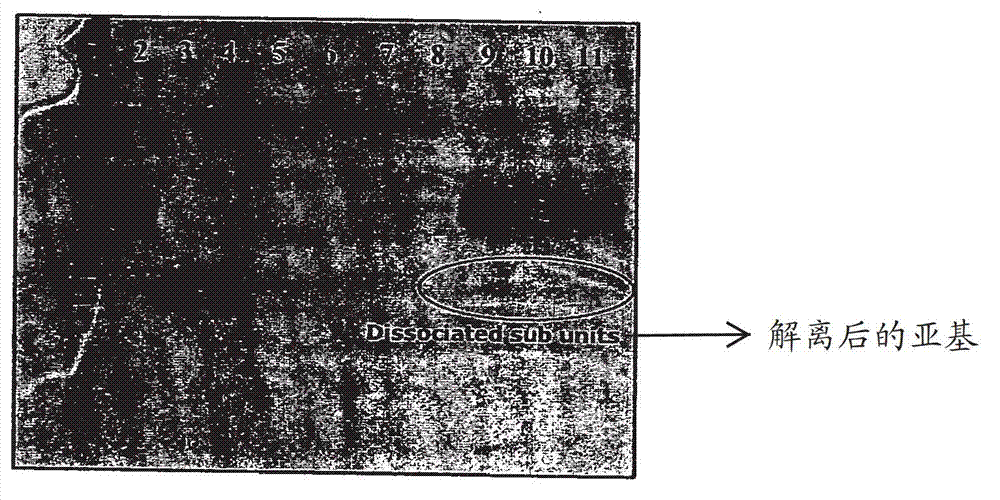
[0086] dissociate
[0087] For the formulations given in Table 1, the percentage of free subunits was assessed by SDS-PAGE. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com