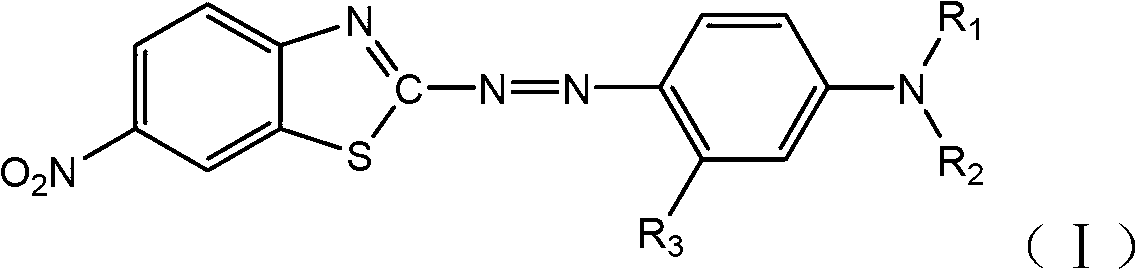
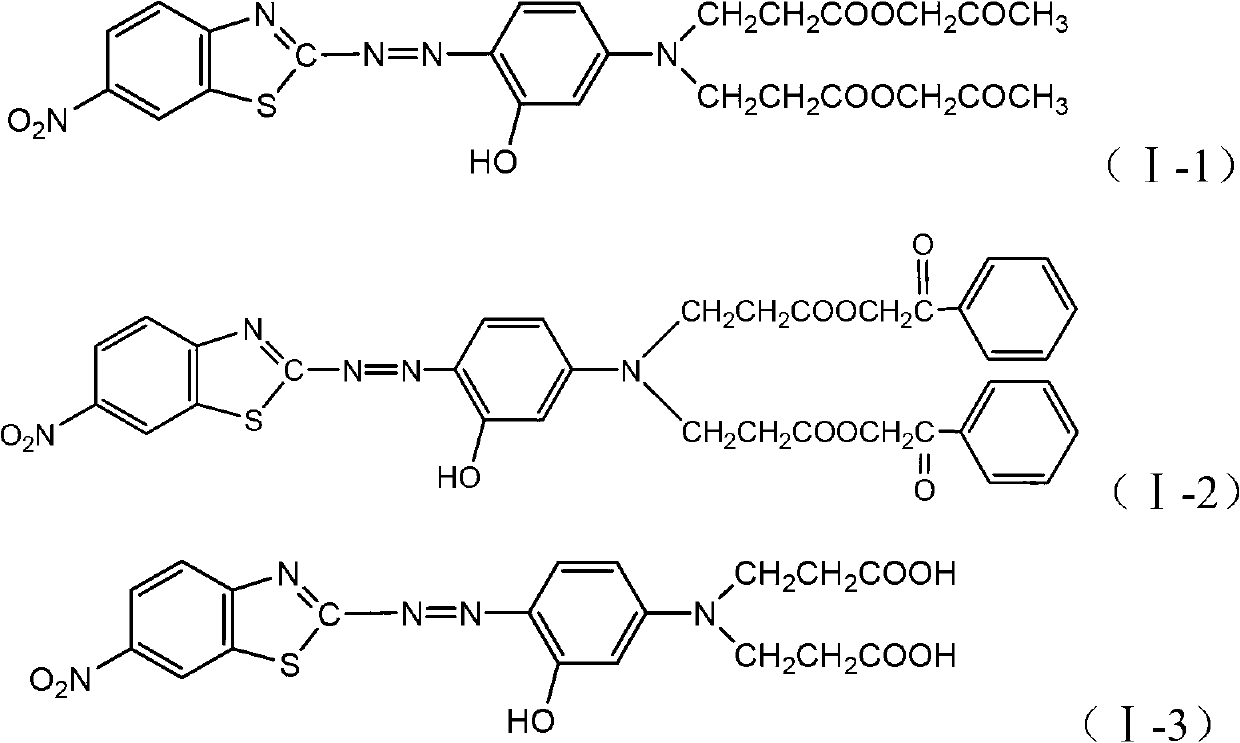
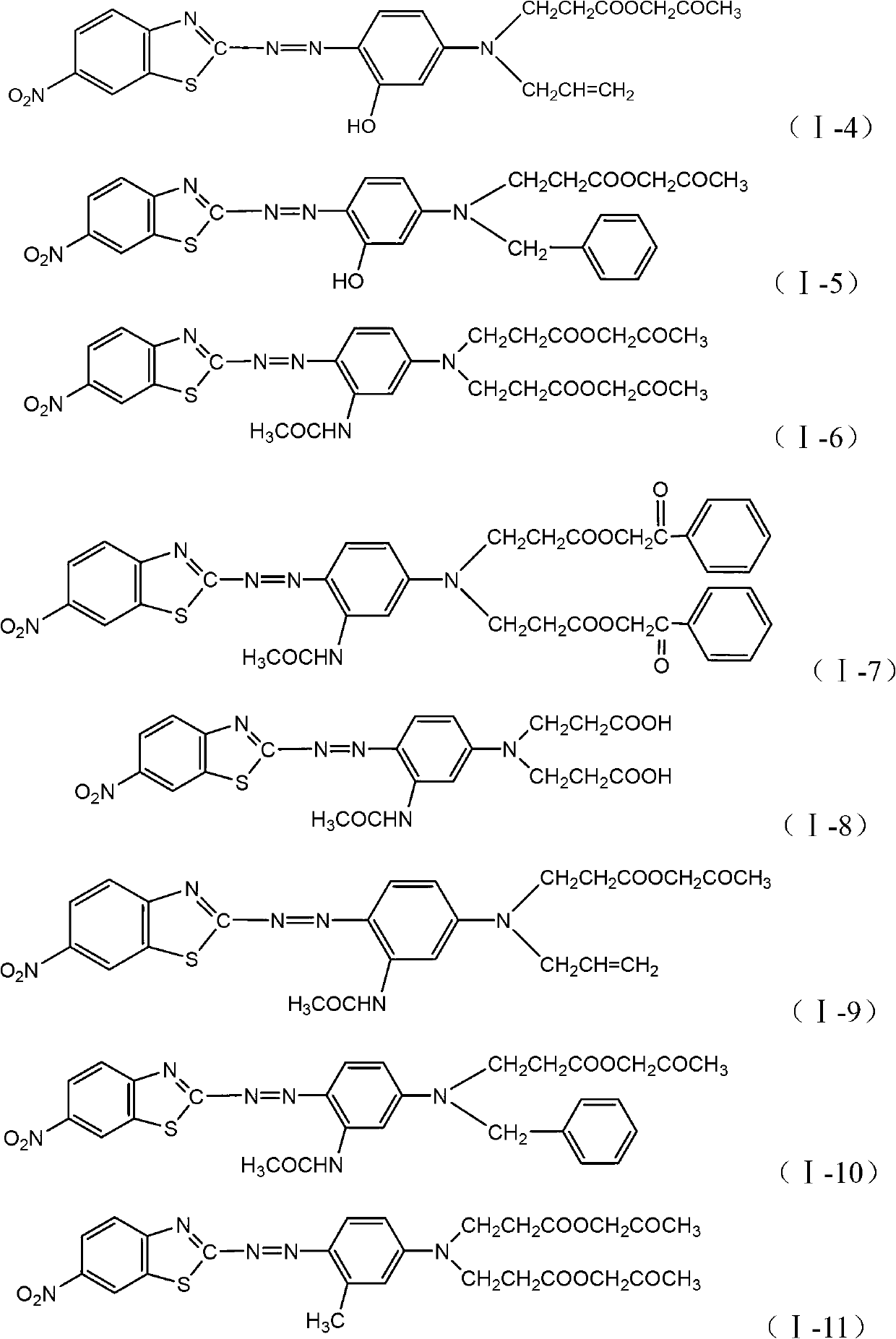
Benzothiazole dye monomeric compound and disperse dye
A technology of benzothiazoles and dye monomers, applied in the direction of azo dyes, organic dyes, monoazo dyes, etc., to achieve uniform color tone, good dyeing depth, and wide dyeing pH range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Put 21.8g (0.2mol) of m-aminophenol, 72g (1mol) of acrylic acid, 100g of water into a 500ml container, heat up to 90°C, keep it warm for 10h, cool down, add 200g of methanol at the same time, keep stirring at 0-5°C for 12h, and crystallize Filter and wash with 50 g of methanol. After washing, dry to obtain the dicarboxylic acid product a.
[0034]
[0035] Put 68.5g (0.5mol) of bromoacetone, 11.6g of soda ash, and dicarboxylic acid product a (100.3g, 0.1mol) into a 500ml container, raise the temperature to 80°C, put in 5gKI, and keep warm for 12h. Cool down to 40°C, add 200g of water, crystallize and filter to obtain coupling component A.
[0036]
[0037] Diazotization: Add 50g of 98% concentrated sulfuric acid, 80g of 85% phosphoric acid, 35g (0.11mol) of 40% nitrosyl sulfuric acid into a 250ml container, cool down to 0~5℃, control 3~4h to finish adding 19.5 g (0.1mol) 2-amino-6-nitrobenzothiazole, after adding, keep warm at 0~5℃ for 3h.
[0038] Coupling: Pu...
Embodiment 2
[0043] Similar to the preparation method of the coupling raw material described in Example 1, the coupling component B can be prepared by replacing bromoacetone with an equimolar amount of 2-bromoacetophenone.
[0044]
[0045] According to the synthesis method of the dye in Example 1, the above-mentioned coupling component B is coupled with the diazo solution of 2-amino-6-nitrobenzothiazole to obtain the finished dye I-2, λ max (DMF)=550nm, the polyester fabric dyed by high temperature and high pressure method is red, the fastness performance is good, the washing fastness reaches grade 5, and the ironing sublimation fastness reaches above grade 4~5.
[0046]
Embodiment 3~12
[0048] According to the preparation methods of Examples 1-2, different coupling raw materials are used to prepare dyes with excellent color fastness properties of the structures shown in Table 1 below (polyester dyeing method is the same as Example 1).
[0049] Table 1
[0050]
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com