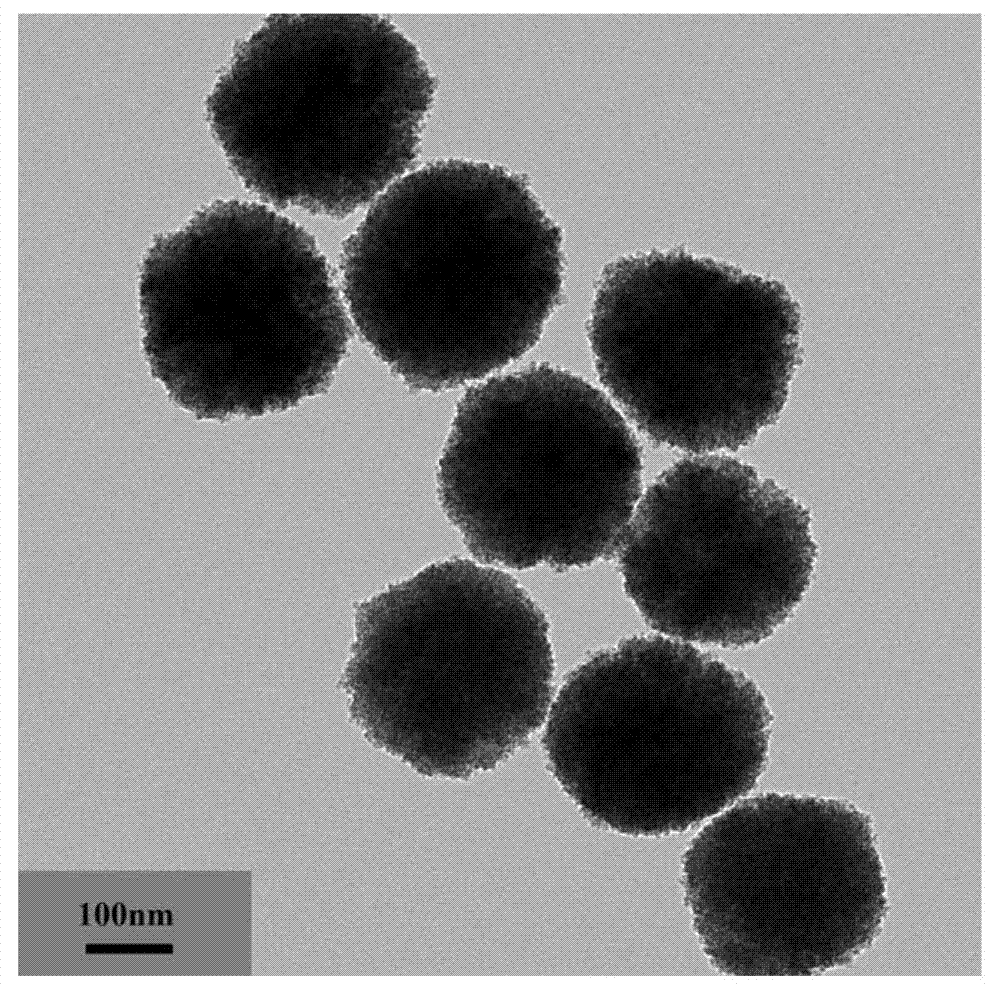
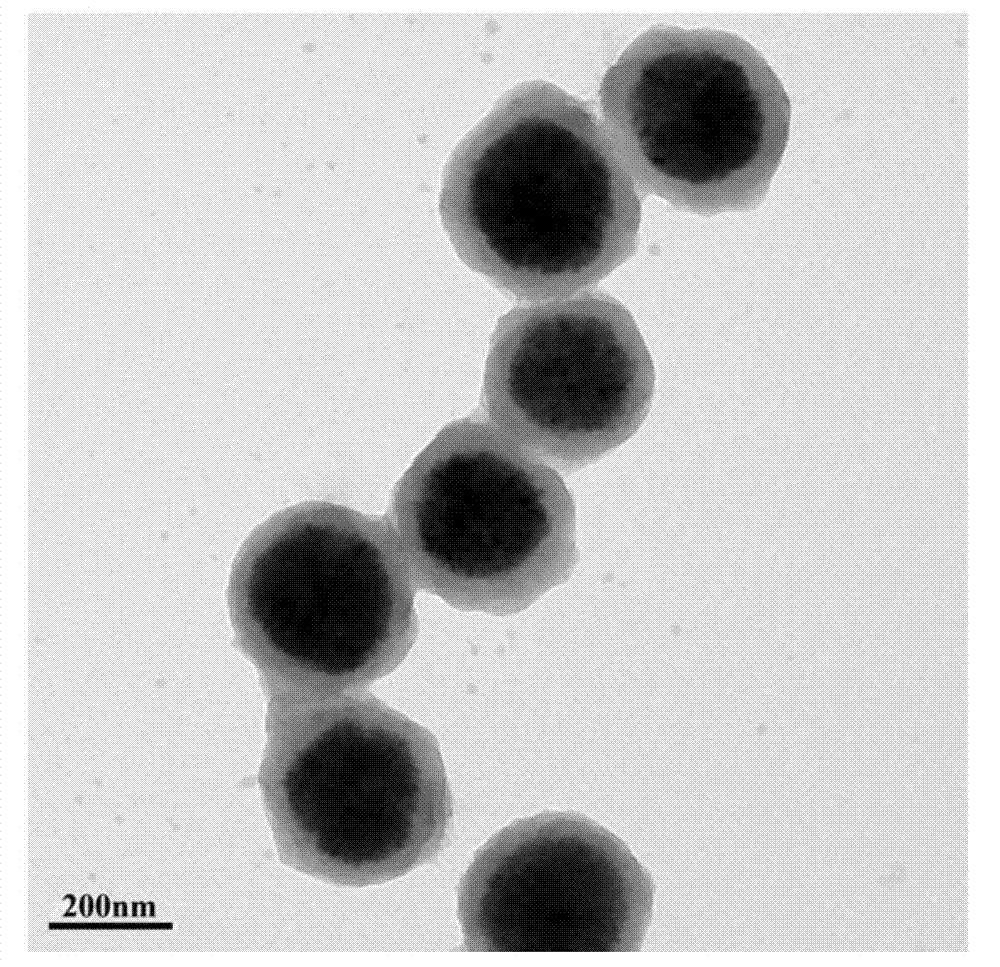
Preparation method of magneitc polymer microspheres for in situ immobilization of noble metal catalyst
A precious metal catalyst, polymer technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of low magnetic content, magnetic polymer microsphere size Problems such as wide distribution to achieve the effect of not easy to drain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0027] 1) Preparation of hydrophilic Fe by solvothermal method 3 o 4 nanoparticles
[0028] 4mmol FeCl 3 ·6H 2 O is completely dissolved in 40ml of ethylene glycol under the action of magnetic stirring, and 0.06 mmol of modifier polyacrylic acid is added under strong magnetic stirring. After it is completely dissolved, 0.11 mol of anhydrous sodium acetate is added to form a khaki precursor solution , and transferred to a 50ml reactor, and reacted at 200°C for 12 hours. The product was washed several times with deionized water and ethanol, and dried under vacuum for 6 hours.
[0029] 2) In situ radical polymerization to prepare Fe 3 o 4 P4VP nanocomposite microspheres
[0030] 0.1g hydrophilic Fe 3 o 4 Ultrasonic dispersion in an aqueous solution containing 0.02mmol of dispersant, and transferred to a 250ml four-necked bottle, after the nitrogen gas was removed to remove the air, the emulsion formed by adding 2.325mmol 4-VP was absorbed by hydrogen bonding under nitrog...
Embodiment example 2
[0034] 1) Preparation of hydrophilic Fe by solvothermal method 3 o 4 nanoparticles
[0035] 4mmol FeCl 3 ·6H 2 O is completely dissolved in 40ml of ethylene glycol under the action of magnetic stirring, and 0.04 mmol of modifier polyacrylic acid is added under strong magnetic stirring. After it is completely dissolved, 0.066 mol of anhydrous sodium acetate is added to form a khaki precursor solution , and transferred to a 50ml reactor, and reacted at 200°C for 12 hours. The product was washed several times with deionized water and ethanol, and dried under vacuum for 6 hours.
[0036] 2) In situ radical polymerization to prepare Fe 3 o 4 P4VP-co-PDVB nanocomposite microspheres
[0037] 0.1g hydrophilic Fe 3 o 4 Ultrasonic dispersion in an aqueous solution containing 0.02mmol dispersant, and transferred to a 250ml four-necked bottle, after purging the air with nitrogen, add the emulsion formed by 4.65 mmol 4-VP and 3.5 mmol DVB, and absorb it through hydrogen bonding un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com