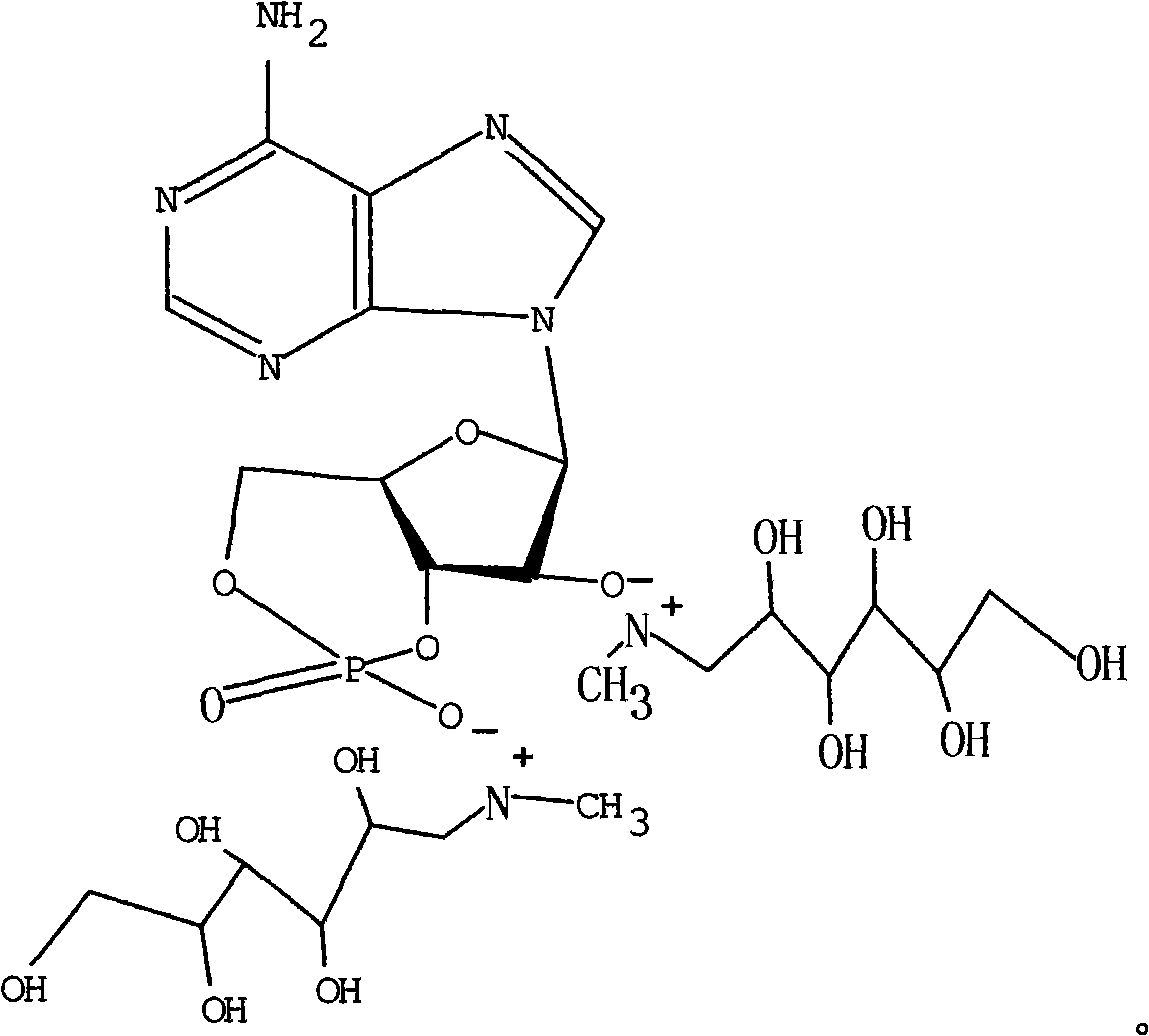
Adenosine cyclophosphate double-molecule meglumine compound and preparation method thereof
A technology of adenosine cyclic monophosphate and meglumine, which is applied in the field of adenosine cyclic monophosphate meglumine compound and its preparation, can solve the problems of increased impurities, inability to use oral preparations, insufficient water solubility, etc., and achieves a simple and convenient preparation method, good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1. cyclic adenosine monophosphate dimeglumine and its preparation
[0025] Add 0.30g (0.911mmol) of cyclic adenosine monophosphate into 20ml of ethanol, stir and heat to completely dissolve, add 0.49g (2.53mmol) of meglumine, slowly add and stir, and heat at 65°C for 1.8 hours after completion , until the solution was clarified, freeze-dried to obtain 0.607 g of adenosine cyclic monophosphate dimglumine as a white powder (yield: 93.5%).
[0026] 1 HNMR (DMSO-d 6 )δ(ppm): 0.767~0.785(4H, t), 1.134~1.160(21H, m), 1.450~1.466(4H, m), 2.514(4H, s), 2.815~2.843(2H, m), 2.946 ~2.997(2H, m), 3.312~3.319(4H, m), 3.385~3.427(8H, m), 3.640~3.654(6H, m), 3.835~3.844(2H, m), 3.931~3.952(4H, m), 4.154 (2H, s), 4.371 (2H, s), 6.658~6.616 (6H, m), 7.082 (2H, d).
[0027] 13 CNMR (DMSO-d 6 )δ (ppm): 13.967, 22.117, 25.597, 29.137, 31.289, 33.289, 51.435, 53.465, 63.461, 66.956, 67.719, 68.864, 70.283, 113.474, 121.044, 130.344, 16.141
Embodiment 2
[0028] Embodiment 2. cyclic adenosine monophosphate dimeglumine and its preparation
[0029] Add 0.50g (1.519mmol) of cyclic adenosine monophosphate into 35ml of water, stir and heat to dissolve completely, add 1.20g (6.148mmol) of meglumine to it, slowly add and stir, and heat at 70°C for 0.8 hours after completion, After the solution was clarified, freeze-dried to obtain 0.987 g of adenosine cyclic monophosphate dimglumine as a white powder (yield: 90.8%).
[0030] The adenosine cyclic monophosphate dimeglumine or its hydrate prepared above was analyzed by H-NMR and C-NMR spectra, which were consistent with those in Example 1.
Embodiment 3
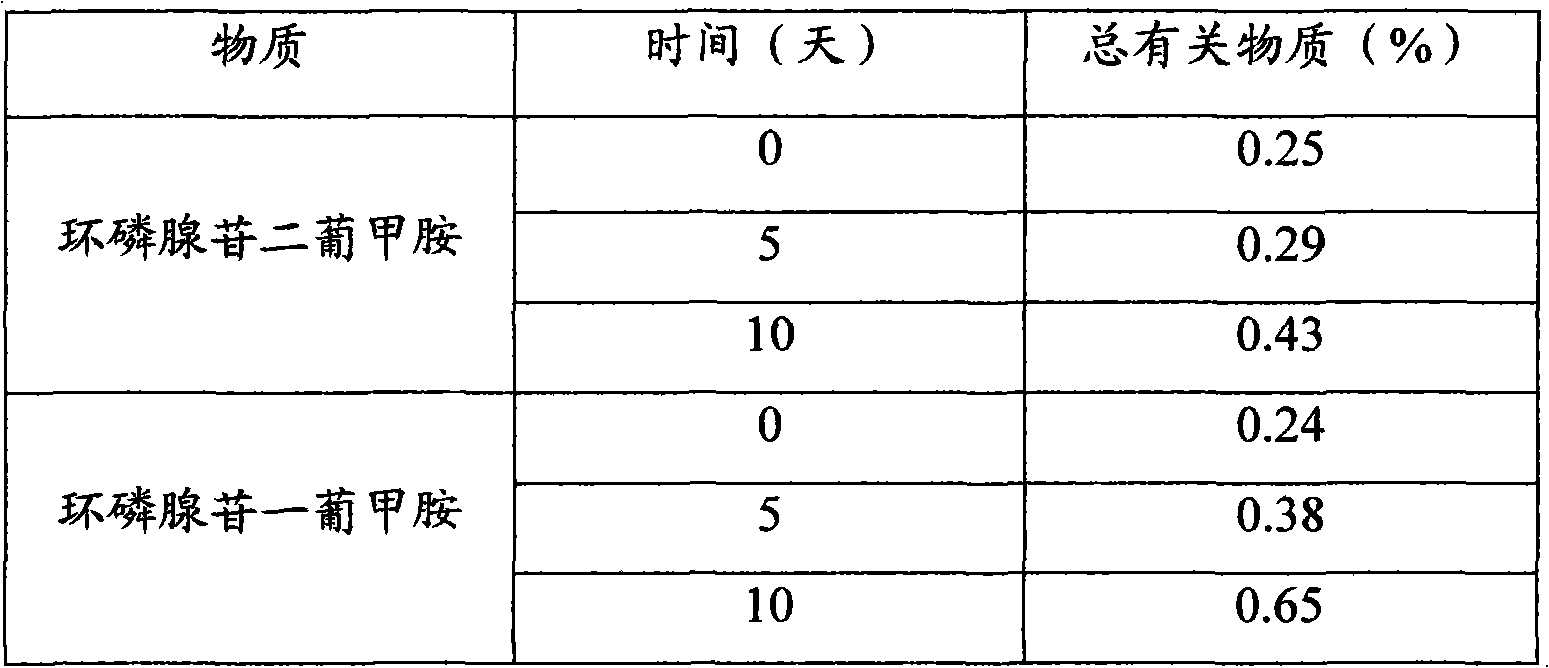
[0031] Embodiment 3. Stability accelerated test
[0032] Cyclic adenosine monophosphate usually decomposes at high temperature to produce impurities, which are collectively referred to as total related substances. Under high temperature conditions, the content of total related substances is determined to analyze its stability.
[0033] Take an appropriate amount of adenosine dimeglumine in a 50°C oven, take samples on the 5th and 10th day respectively, and determine the total related substances therein.
[0034] It was analyzed by high performance liquid chromatography, and the test results are shown in Table 1.
[0035] Table 1
[0036]
[0037] The measurement results showed that the content of total related substances increased when adenosine dimglumine was placed at a high temperature of 50°C for 10 days, but most of them remained stable, which was more stable than adenosine meglumine cyclophosphate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com