Recombinant expression plasmid and application thereof in preparing anti-tumor immunogene therapeutic medicament
A technology of anti-tumor immunity and expression plasmid, applied in the field of biopharmaceuticals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
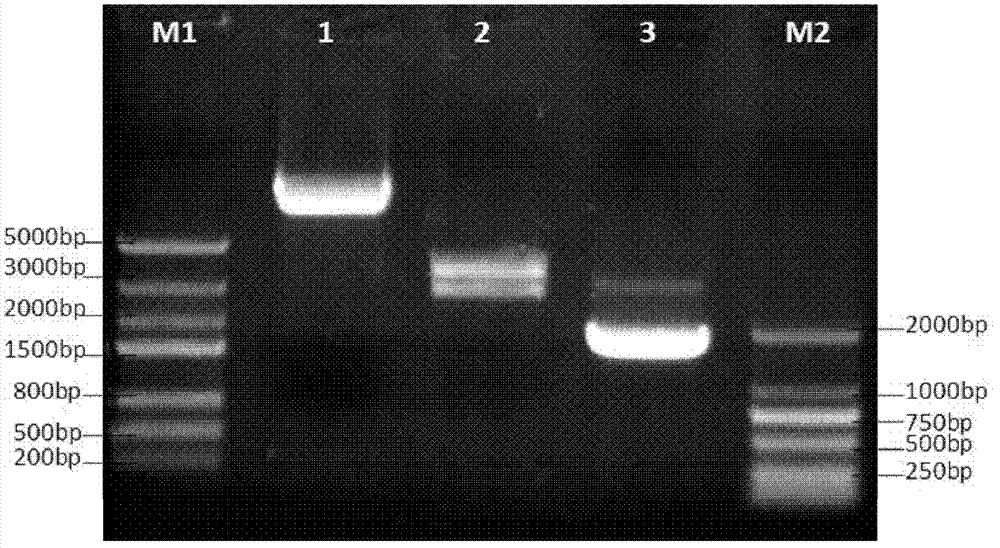
[0023] Embodiment 1, preparation of recombinant expression plasmid pVAX-IL-12-GB
[0024] The active ingredient of the anti-tumor immune gene therapy drug of the present invention is the recombinant expression plasmid pVAX-IL-12-GB carrying the human immunoregulatory factor IL-12 gene, GM-CSF gene and B7.1 gene. In this plasmid, human immune The positions of the regulatory factors IL-12 gene, GM-CSF gene and B7.1 gene in the carrier are human immune regulatory factor IL-12 gene, GM-CSF gene and B7.1 gene from upstream to downstream, and the GM- The CSF gene and the B7.1 gene are connected through a linker to form a fusion gene (GM-CSF-B7.1), and connected to the IL-12 gene through the IRES sequence, that is, the IL-12 gene from the upstream of the IRES, and the fusion gene from the downstream of the IRES GM-CSF-B7.1.
[0025] The plasmid construction method is as follows:
[0026] The pCI-IL-12 plasmid containing the human IL-12 gene (see the literature for the construction ...
Embodiment 2
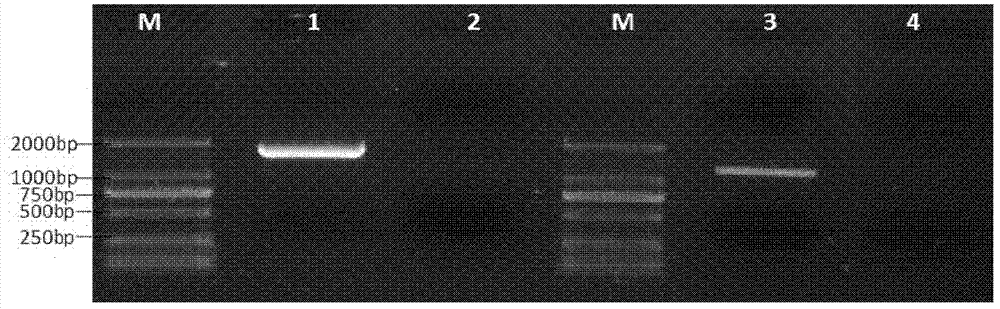
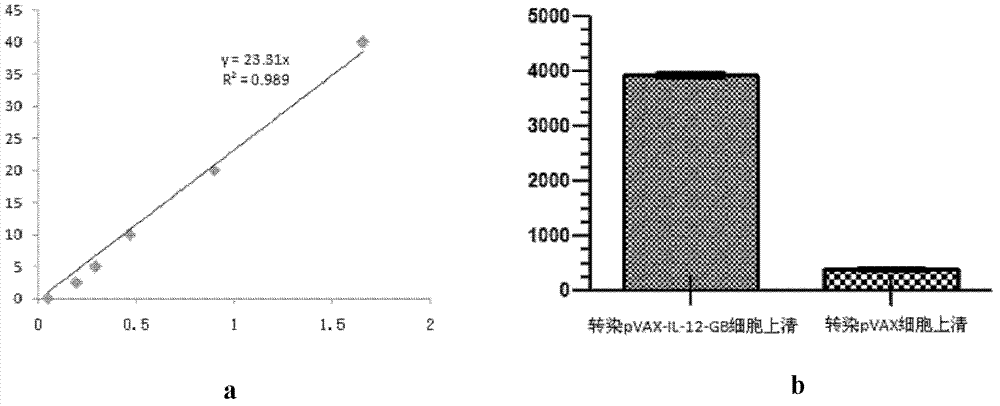
[0030] Example 2, detection of expression levels of IL-12 gene and GM-CSF-B7.1 fusion gene in cells transfected with plasmid pVAX-IL-12-GB
[0031] 1. Transfection of pVAX-IL-12-GB recombinant expression plasmid into 293T cells
[0032] Inoculate the positive clones transfected with the pVAX-IL-12-GB recombinant expression plasmid in 200mL LB liquid medium, culture at 37°C and shake at 200rpm for 12 hours, and extract a large amount of pVAX-IL-12-GB ultrapure plasmid (Use Tiangen Biochemical Technology Company’s endotoxin-free plasmid mass extraction kit), take 293T cells in the logarithmic growth phase and divide them into six-well plates one day in advance, and when they grow to 60%-70% on the second day, transfect Reagent Lipofectamine 2000 (Invitrogen Company) transfected 293T cells with pVAX-IL-12-GB ultrapure plasmid, and replaced 1640 complete medium (product of Gbico Company) with 10% fetal bovine serum after 6 hours to transfect pVAX1 empty vector Plasmids (products ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com