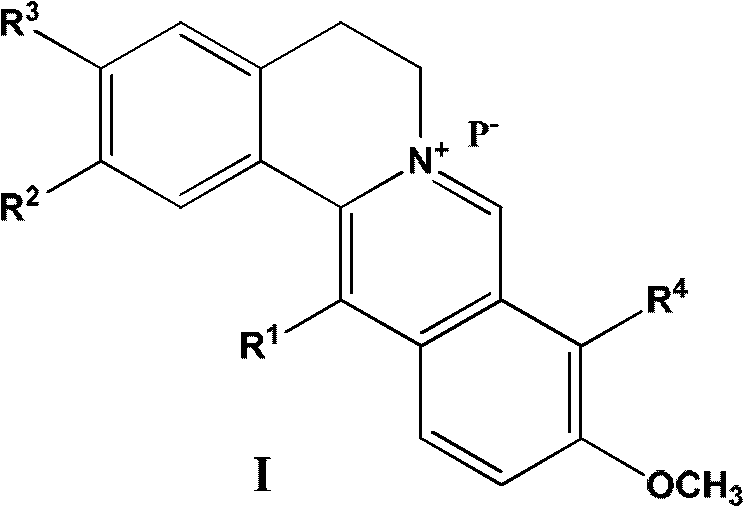
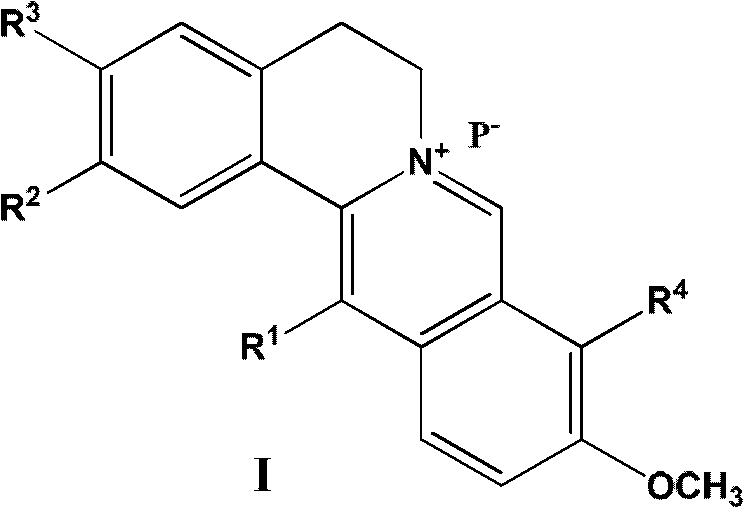
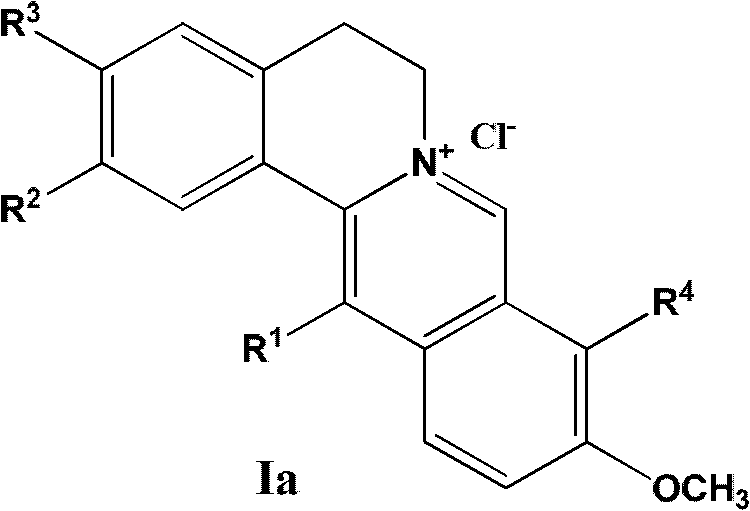
13-substituted berberine derivatives and preparation method thereof, and uses of 13-substituted berberine derivatives as anti-tuberculosis drugs
A kind of berberine, 10-technology, applied in the field of medicinal chemistry, can solve the problem of anti-tuberculosis candidates with no new structure skeleton, since the mid-1970s, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] Example 1: Synthesis of 2,3-methylenedioxy-9,10-dimethoxy-13-n-octyl orthoberberine chloride (Y-191)
[0134] A solution of 5% sodium hydroxide (10 ml) dissolved in sodium borohydride (0.80 g, 21 mmol) was added dropwise to methanol (250 ml) containing berberine (7.43 g, 20 mmol) and potassium carbonate (8.3 g, 60 mmol). ) solution system, stirred at room temperature for two hours, collected the precipitated dark green solid by suction filtration, washed the filter cake several times with water, and recrystallized with 95% ethanol to obtain the intermediate dihydroberberine.
[0135] The intermediate dihydroberberine (5.0g, 15mmol) was dissolved in 80% ethanol (200ml), then 10ml of n-octanal and 50ml of acetic acid were added successively, heated to 85~95°C for reflux for 5 hours, and the reaction solution was reduced Concentrate under reduced pressure to obtain dark red oil, soak in ether for a period of time, filter with suction, collect the ether layer, add 2% hydroc...
Embodiment 2
[0139] Example 2: Synthesis of 2,3-methylenedioxy-9-hydroxyl-10-methoxy-13-n-octyl protoberberine chloride (A-18)
[0140]Put Y-191 (1.2g, 2.48mmol) in a 250ml flask, keep the vacuum at 30-40mmHg, heat to 195-210°C and react for 10-15min. It is found that the color of the solid changes from yellow to deep red quickly. After the reaction was completed, concentrated hydrochloric acid: ethanol (5:95) was acidified for recrystallization, but no solid crystallized out, and evaporated to dryness to obtain 1.14 g of solid, yield: 98%. mp 122-124°C.
[0141] MS-ESI(M / Z): 434.2【M-Cl】 +
[0142] 1 H-NMR (CD 3 OD, δppm): 0.85(t, J=7.2Hz, 3H), 1.23~1.41(m, 10H), 1.82(s, 2H), 3.04(t, J=6.0Hz, 2H), 3.33(t, J =8.4Hz, 2H), 4.03(s, 3H), 4.67((t, J=6.0Hz, 2H), 6.06(s, 2H), 6.96(s, 1H), 7.23(s, 1H), 7.79( d, J=9.2Hz, 1H), 7.96(d, J=9.2Hz, 1H), 9.72(s, 1H); 13 C NMR (DMSO-d6) δ: 148.8, 146.5, 145.2, 144.6, 144.4, 134.8, 133.9, 133.6, 131.6, 124.6, 120.5, 117.2, 115.9, 109.1, 108.3, 102.0, ...
Embodiment 3
[0144] Example 3: Synthesis of 2,3-methylenedioxy-9-ethoxy-10-methoxy-13-n-octyl protoberberine chloride (B-7)
[0145] Dissolve A-18 (200mg, 0.43mmol) in DMF (10ml), add finely ground KOH (78.4mg, 1.4mmol) and bromoethane (37.3μl, 0.5mmol), stir at room temperature for about one day, and concentrate under reduced pressure The solvent was removed, acidified with dilute hydrochloric acid, and finally analyzed and purified by vacuum silica gel column to obtain 82 mg of pure product, yield: 35.7%. mp 86-88°C.
[0146] MS-ESI(M / Z): 462.0【M-Cl】 +
[0147] 1 H-NMR (CD 3 OD, δppm): 0.84(t, J=7.2Hz, 3H), 1.24~1.48(m, 13H), 1.81(t, J=7.6Hz, 2H), 3.06(t, J=6.0Hz, 2H), 3.37(t, J=8.0Hz, 2H), 4.06(s, 3H), 4.43(q, J=6.8Hz, 2H), 4.74(t, J=6.0Hz, 2H), 6.07(s, 2H), 6.98(s, 1H), 7.24(s, 1H), 8.10(d, J=9.2Hz, 2H), 9.67(s, 1H); 13 C NMR (CD 3 OD)δ: 151.8, 151.3, 148.7, 145.3, 145.1, 137.7, 136.3, 135.1, 134.4, 127.0, 123.5, 122.1, 121.8, 110.4, 109.3, 103.7, 71.5, 58.9, 57.5, 32.3, 30.9, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com