Method for preparing substituted indole-3-methanal compound
A technology of compound and compound structural formula, which is applied in the field of preparation of substituted indole-3-carboxaldehyde compounds, can solve problems such as industrialization difficulties and operational difficulties, and achieve the effects of reducing side reactions, increasing reaction yield, and reducing adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
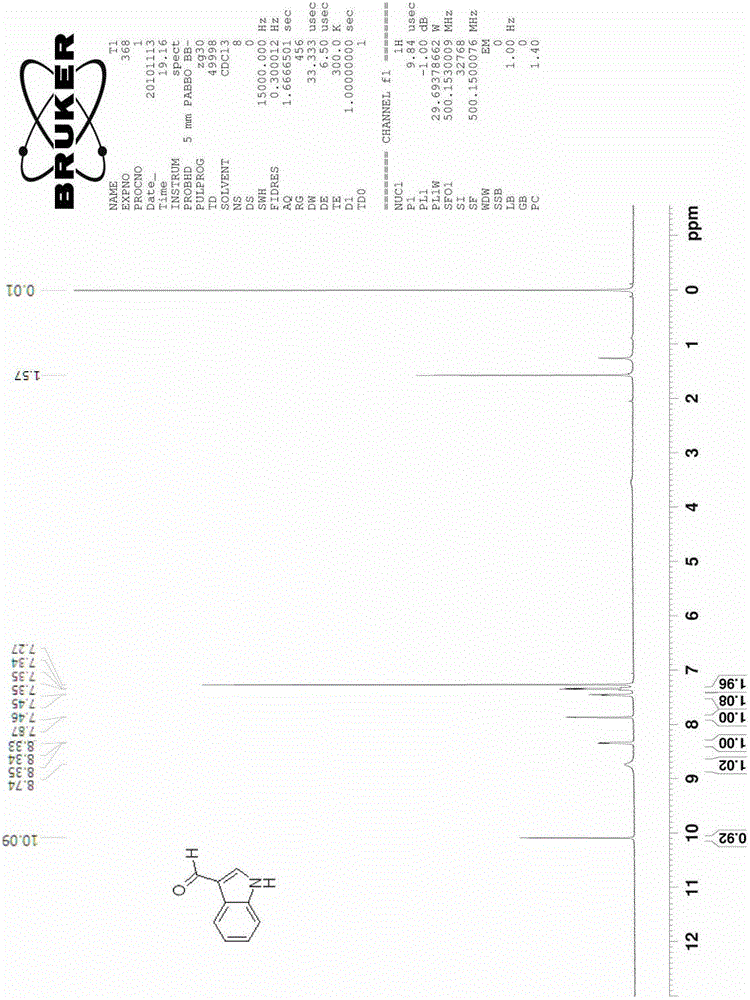
[0030] The synthesis of embodiment 1 indole-3 formaldehyde
[0031]
[0032] Add 5.6 g of 2-nitrotoluene (compound 1a, 0.041 mol) and 24.3 g of DMFDMA (0.205 mol) into 80 ml of acetonitrile, raise the reaction temperature to 85°C-95°C, and reflux for 5 hours. The reaction solution was cooled, the solvent was spin-dried, and washed with water and a small amount of ethanol to obtain 7.7 g of crude product 2a (0.0402 mol, yield 98.0%).
[0033] The obtained compound 2a was added to 130ml of methanol containing 10wt% hydrazine (hydrazine content 0.4mol, 10eq), and the temperature was raised to 50°C, reacted for 8 hours, and cooled to room temperature. The solvent was spin-dried to obtain product 3a (4.5 g, 0.0385 mol, yield 96.1%), which was dissolved in 20 ml of acetonitrile.
[0034] Add 11.2 grams of DMF (0.154mol, 4eq) and 3 grams of 4A molecular sieve into 100ml of acetonitrile, and cool the reaction to 0°C to 5°C, slowly add 11.6g of POCl dropwise under ice bath conditio...
Embodiment 2
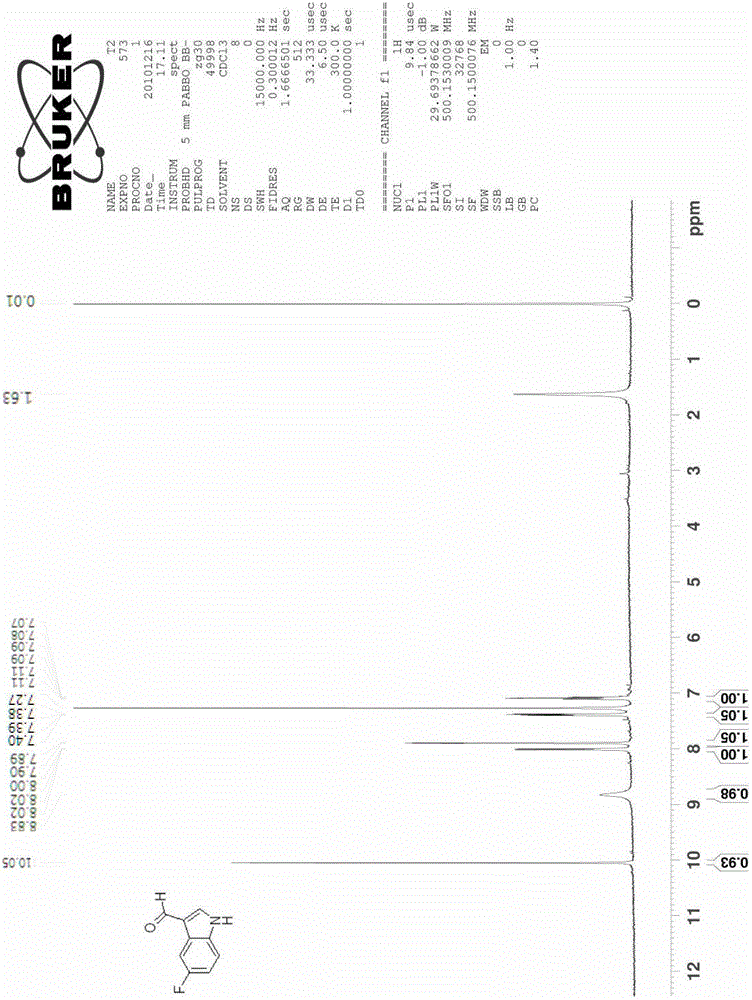
[0037] Example 2 Synthesis of 5-fluoroindole-3-carbaldehyde
[0038]
[0039] Add 7.2 g of 5-fluoro-2-nitrotoluene (compound 1b, 0.046 mol) and 27.4 g of DMFDMA (0.232 mol) into 80 ml of acetonitrile, raise the reaction temperature to 85°C-95°C, and reflux for 5 hours. The reaction was cooled, the solvent was spin-dried, and washed with water and a small amount of ethanol to obtain 9.3 g of crude product 2b (0.0443 mol, yield 96.2%).
[0040] The obtained intermediate 2b was added to 147ml of methanol containing 10wt% hydrazine (hydrazine content 0.45mol, 10eq), and the temperature was raised to 50°C, reacted for 8 hours, and cooled to room temperature. The solvent was spin-dried to obtain 5.7 g of product 3b (0.0424 mol, yield 95.8%), which was dissolved in 20 ml of acetonitrile.
[0041] Add 12.3 grams of DMF (0.168mol, 4eq) and 3.3 grams of 4A molecular sieve into 100ml of acetonitrile, and cool the reaction to 0°C-5°C, slowly add 12.8g of POCl dropwise under ice bath c...
Embodiment 3
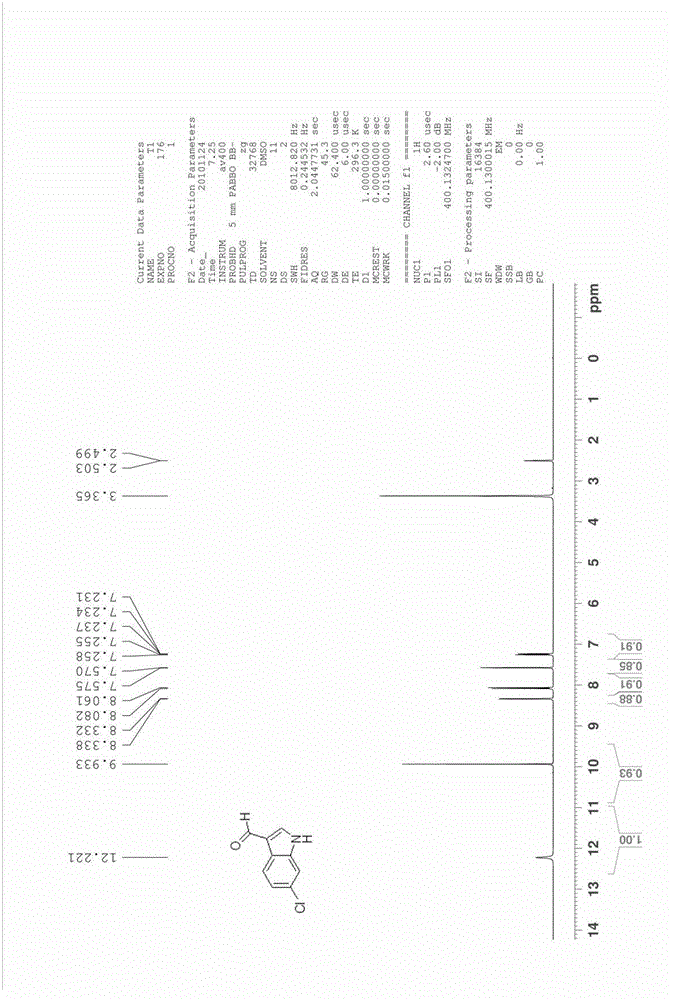
[0043] The synthesis of embodiment 3 6-chloroindole-3-formaldehyde
[0044]
[0045] Add 6.6 g of 4-chloro-2-nitrotoluene (compound 1c, 0.039 mol) and 23.0 g of DMFDMA (0.193 mol, 5 eq) into acetonitrile (80 ml), raise the reaction temperature to 85°C to 95°C, and reflux for 5 hours . The reaction was cooled, the solvent was spin-dried, and washed with water and a small amount of ethanol to obtain 8.4 g of crude product 2c (0.0369 mol, yield 94.6%).
[0046] The resulting intermediate 2c was added to 118ml of methanol containing 10wt% hydrazine (hydrazine content: 0.36mol, 10eq), heated to 50°C, reacted for 8 hours, and cooled to room temperature. The solvent was spin-dried to obtain 5.4 g of product 3c (0.0355 mol, yield 96.3%), which was dissolved in 20 ml of acetonitrile.
[0047] Add 10.4 grams of DMF (0.142mol, 4eq) and 3 grams of 4A molecular sieves into acetonitrile (100ml), and cool the reaction to 0°C to 5°C, slowly add 10.8g of POCl dropwise under ice bath condi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com