Preparation method of lurasidone intermediate and lurasidone
A lurasidone and body type technology, applied in the preparation of sulfonates, organic chemistry and other directions, can solve the problems of long reaction time, high cost, troublesome post-processing, etc., and achieve short reaction time, low cost and simple post-processing. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
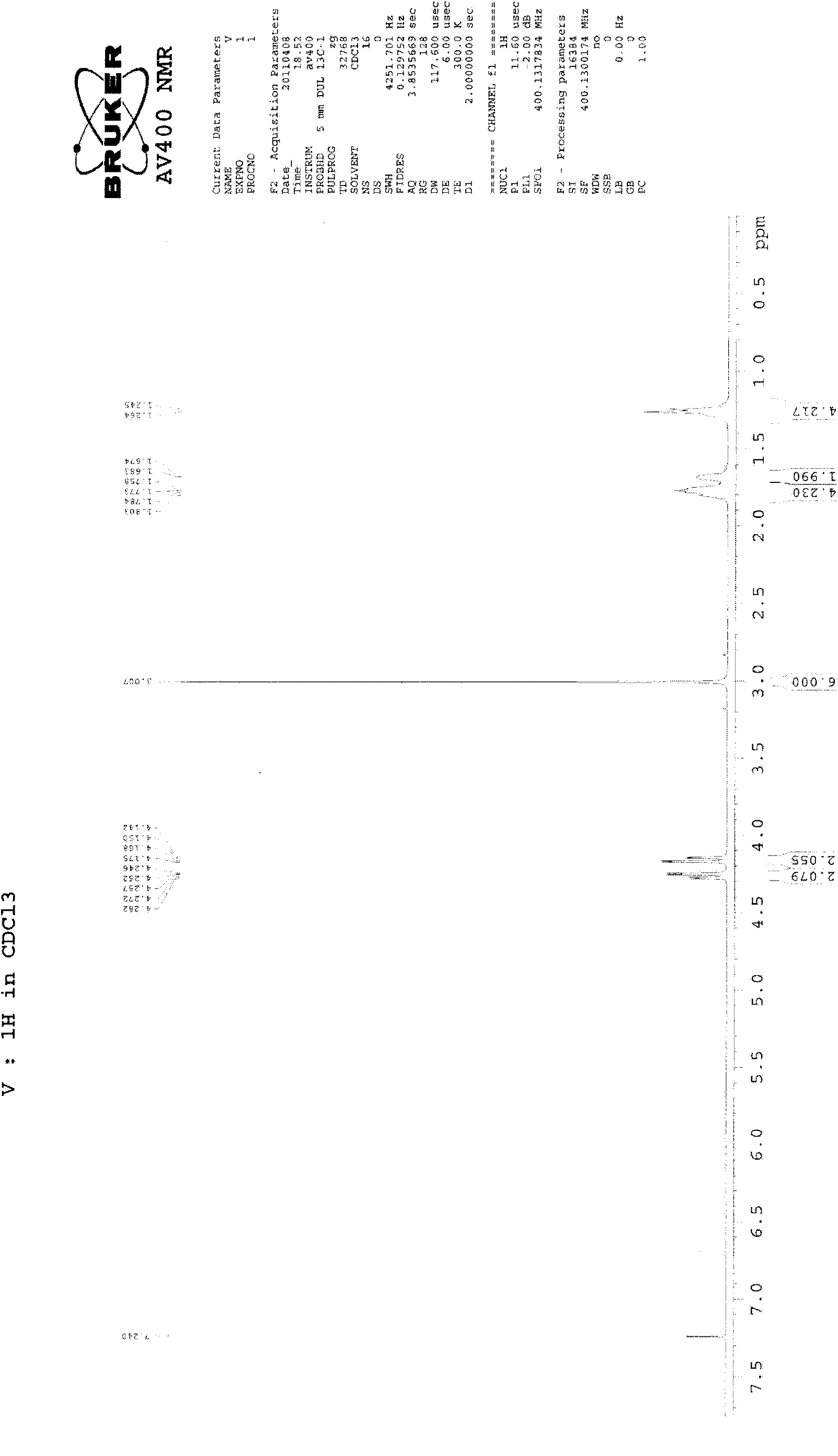
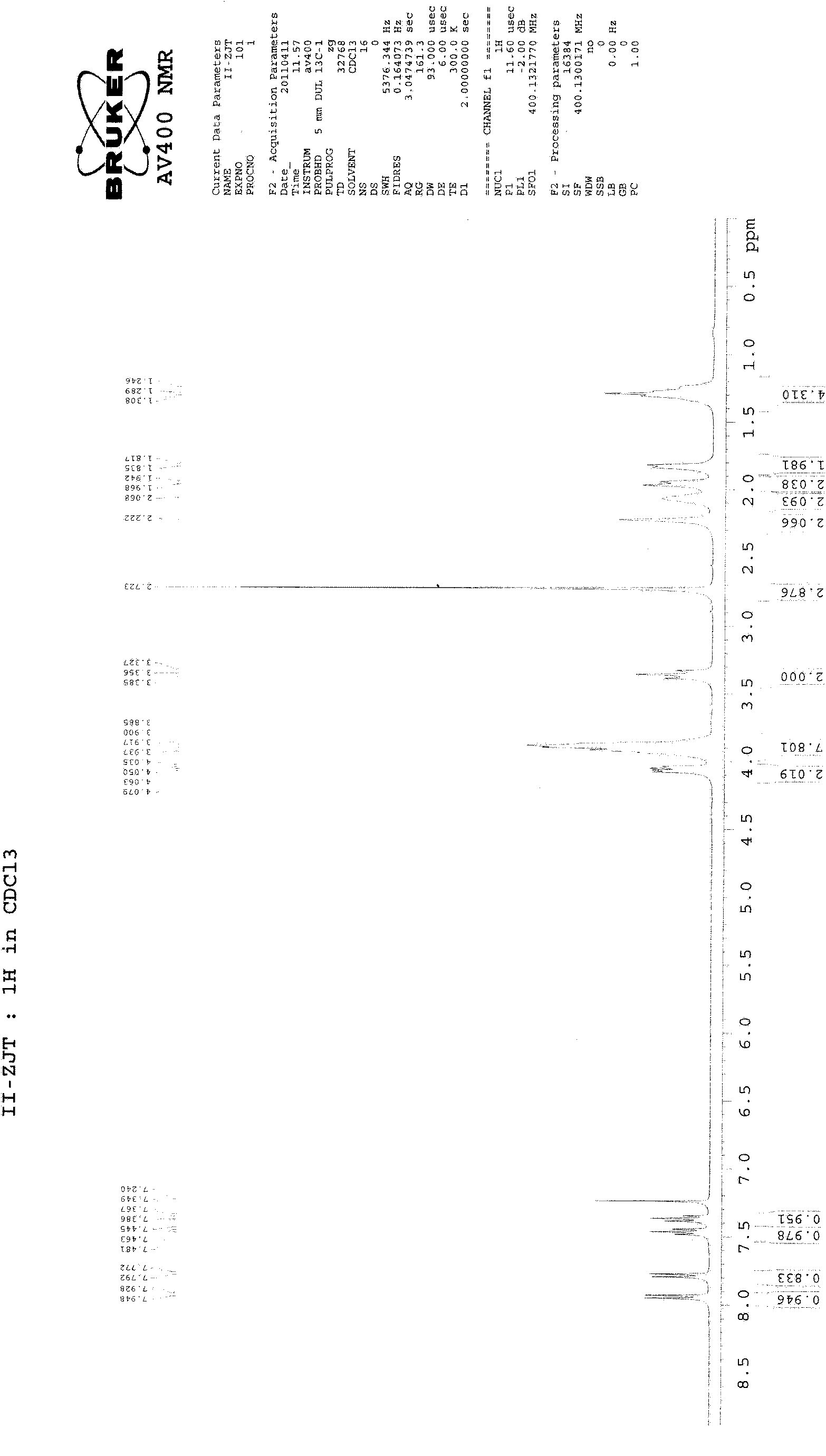
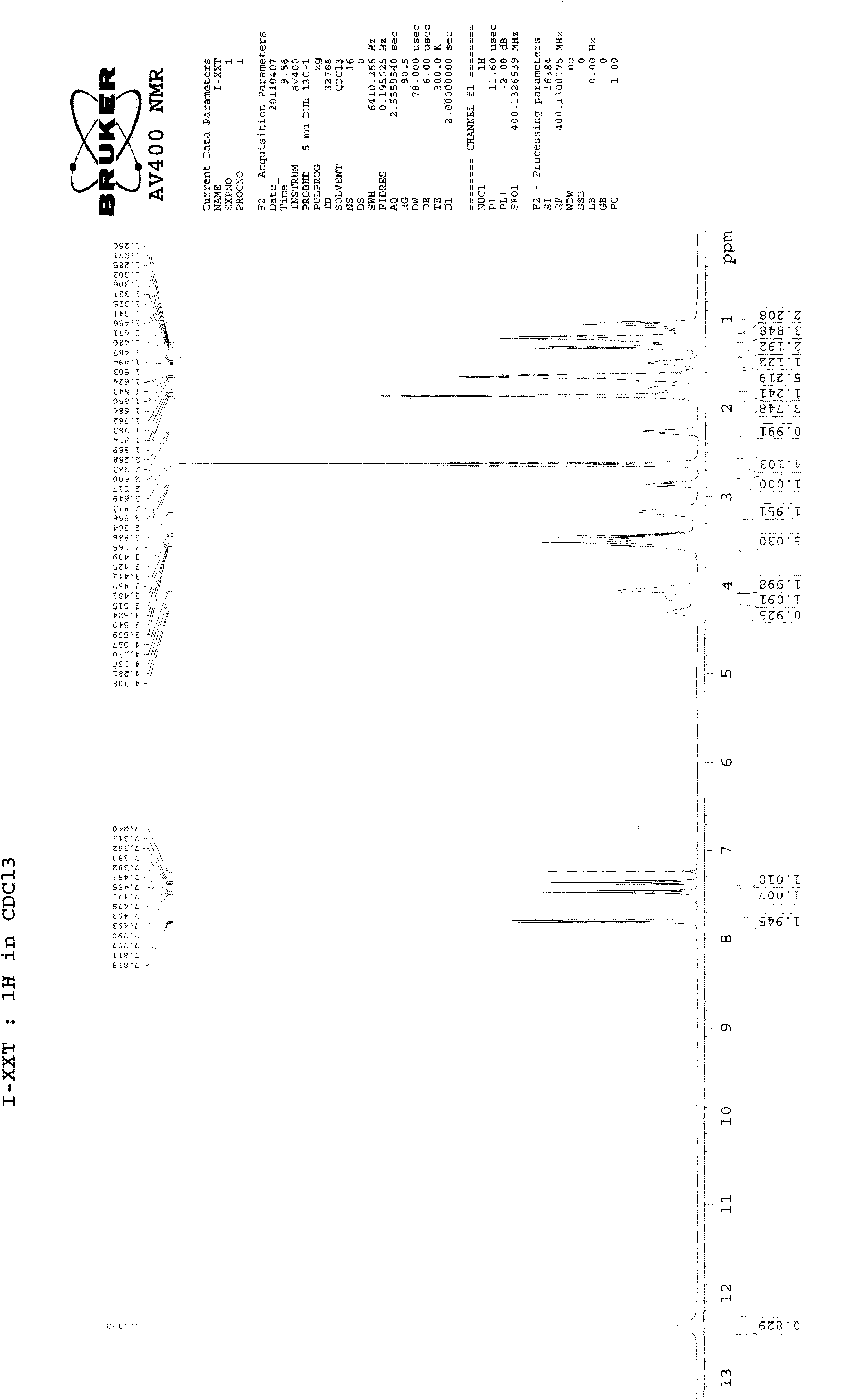
[0028] 27g of compound of formula (Ⅴ), 18g of compound of formula (Ⅳ), 10g of potassium carbonate (ground and sieved, average particle size 198 microns), 1g of tetrabutylammonium bromide and 300ml of toluene are added in the reaction flask equipped with water separator , heated to reflux to separate water, and after 12 hours, the TLC plate was spotted, and the raw materials basically disappeared. Stop the reaction, filter while hot, evaporate the filtrate to dryness, and recrystallize from acetonitrile to obtain 26 g of the compound of formula (II), with a yield of 75% and a melting point of 228-230°C. 1 H-NMR (CDCl 3 )δ: 1.25-1.31 (4H, m), 1.82-2.22 (8H, m), 2.72 (3H, s), 3.36 (2H, t), 3.88-4.08 (10H, m), 7.37 (1H, t) , 7.46(1H, t), 7.78(1H, d), 7.94(1H, d) (see figure 2 ).
Embodiment 2
[0030] 27g of compound of formula (Ⅴ), 18g of compound of formula (Ⅳ), 8g of sodium carbonate (grinding and sieving, average particle diameter of 198 microns), 1g of tetrabutylammonium bromide and 300ml of toluene are added in the reaction flask equipped with water separator , heated to reflux to separate water, and after 11 hours, the TLC plate was spotted, and the raw materials basically disappeared. Stop the reaction, filter while hot, evaporate the filtrate to dryness, and recrystallize from acetonitrile to obtain 25.8 g of the compound of formula (II), with a yield of 74% and a melting point of 228-230°C. 1 H-NMR (CDCl 3 )δ: 1.25-1.31 (4H, m), 1.82-2.22 (8H, m), 2.72 (3H, s), 3.36 (2H, t), 3.88-4.08 (10H, m), 7.37 (1H, t) , 7.46(1H, t), 7.78(1H, d), 7.94(1H, d) (see figure 2 ).
Embodiment 3
[0032] 27g of compound of formula (Ⅴ), 18g of compound of formula (Ⅳ), 10g of potassium carbonate (ground and sieved, average particle diameter of 165 microns), 1g of tetrabutylammonium bisulfate and 300ml of toluene are added in the reaction flask equipped with water separator , heated to reflux to separate water, and after 10 hours, the TLC plate was spotted, and the raw materials basically disappeared. Stop the reaction, filter while hot, evaporate the filtrate to dryness, and recrystallize from acetonitrile to obtain 26.4 g of the compound of formula (II), with a yield of 76% and a melting point of 228.5-230.5°C. 1 H-NMR (CDCl 3 )δ: 1.25-1.31 (4H, m), 1.82-2.22 (8H, m), 2.72 (3H, s), 3.36 (2H, t), 3.88-4.08 (10H, m), 7.37 (1H, t) , 7.46(1H, t), 7.78(1H, d), 7.94(1H, d) (see figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com