Cationic macromolecular proteolipid gene medicine carrier, preparation method and application
A gene drug and macromolecule technology, which can be used in inactive components of polymer compounds, gene therapy, drug combinations, etc., can solve the problems of high cytotoxicity, poor tumor targeting, and low transfection efficiency, and achieve simple preparation and operation. , reversal of drug resistance characteristics, strong tumor targeting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
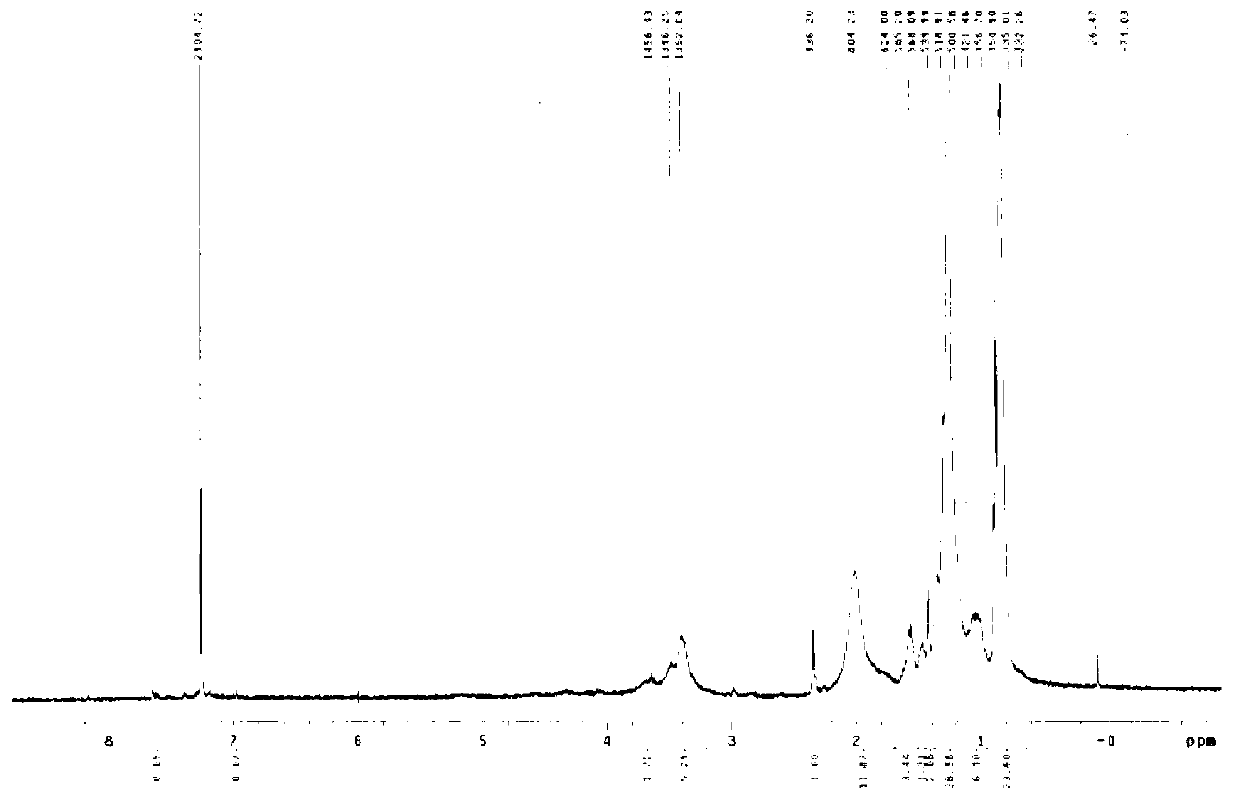
[0044] Embodiment 1: This embodiment discusses the preparation method of transferrin-long chain alkyl quaternary ammonium salt:
[0045] Dissolve 10 mg of transferrin (Tf) in 10 ml of a mixed solution of deionized water and isopropanol (the volume ratio of water and isopropanol is 1:1); Ammonium chloride 100 mg was slowly added to the system; after stirring at room temperature for 24 hours, the reaction solution was dialyzed with deionized water for 3 days, and freeze-dried to obtain transferrin-hexadecyl quaternary ammonium salt (Tf-HQ) White powder 12.0mg.
[0046] Among them, dimethyl hexadecyl epoxypropyl ammonium chloride can be replaced by other long-chain alkyl quaternary ammonium salts, and other long-chain alkyl quaternary ammonium salts include dimethyl octadecyl epoxypropyl ammonium chloride Ammonium, Dimethylbehenylglycidyl ammonium chloride, Dimethyltetradecylglycidyl ammonium chloride, Dimethyldodecylglycidyl ammonium chloride.
Embodiment 2
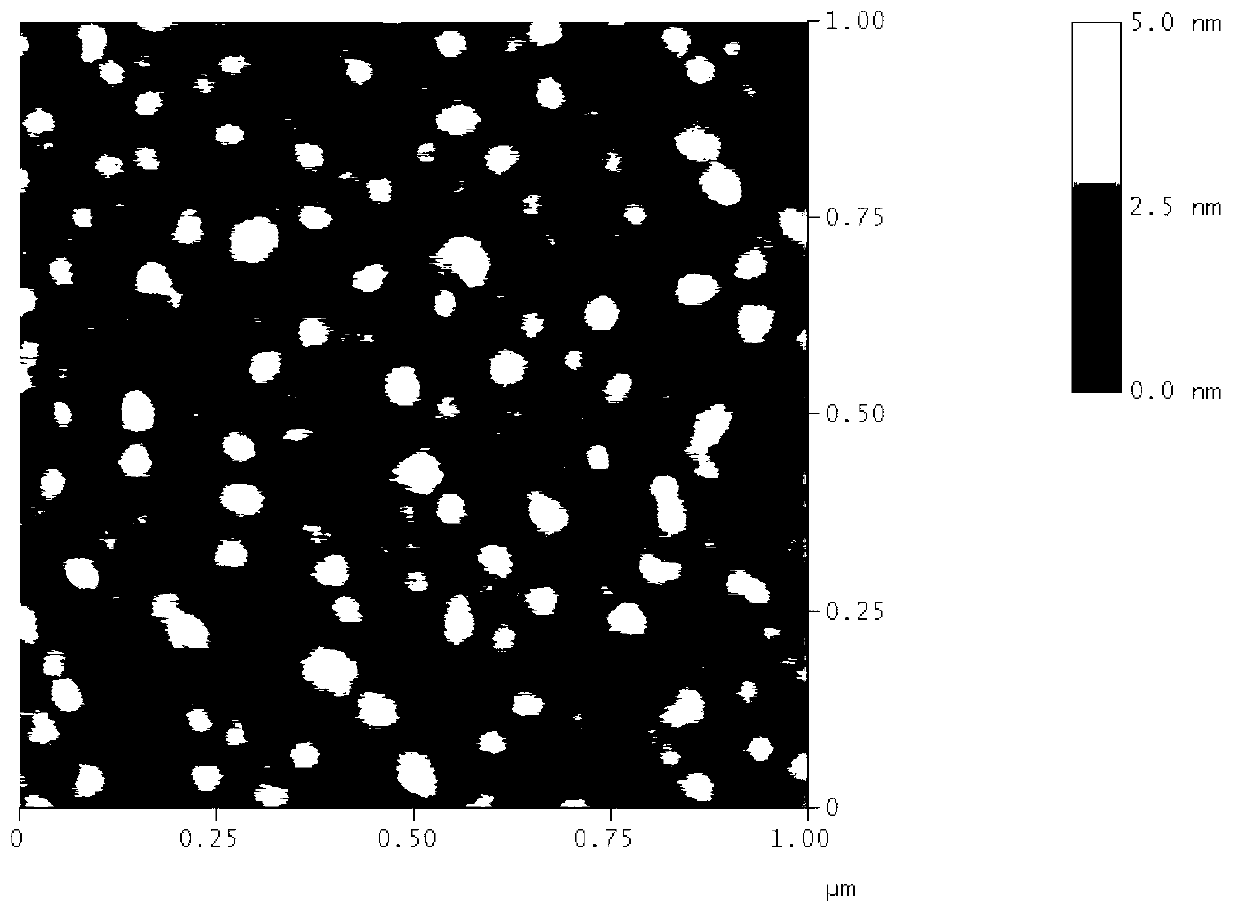
[0048] This example discusses the effect of different ratios of transferrin-hexadecyl quaternary ammonium salt (Tf-HQ), tetradecyl carboxymethyl chitosan quaternary ammonium salt (TQCMC) and cholesterol on cations when the reverse evaporation method is adopted. Effect of particle size on macromolecular transferrin liposomes.
[0049] Co-dissolve Tf-HQ (protein molecular weight: 79,000), TQCMC and cholesterol in different proportions in dichloromethane, and mix well to obtain solution I; prepare deionized aqueous solution II, in which the ratio of solution I and solution II is 1:2 After the two solutions are mixed, fully ultrasonic emulsification, dichloromethane is distilled off under reduced pressure on a rotary evaporator to obtain a transferrin liposome solution. As can be seen from Table 2, adjusting the mass ratio of Tf-HQ and cholesterol can obtain transferrin liposomes of different particle sizes, and the particle size distribution of the cationic transferrin liposomes ...
Embodiment 3
[0054] This example mainly provides an example of preparing cationic transferrin liposomes using other lipid components.
[0055] Weigh transferrin-hexadecyl quaternary ammonium salt (Tf-HQ) (quaternary ammonium salt substitution degree is 90.0%, protein molecular weight is 79,000) 15.0mg, DOPE 12.0mg are dissolved in 3.0ml dichloromethane, Oscillate evenly to obtain solution I, put it into an eggplant-shaped bottle, distill it under reduced pressure on a rotary evaporator, and pass nitrogen gas from time to time until the dichloromethane is completely evaporated, then dry it in vacuum at room temperature for 24 hours, and then use it with 3.0 mg of water-soluble 5.0mL PBS (pH=7.4) buffer solution II of magnetic particles was ultrasonically hydrated for 10min (thin film method); or the above-mentioned solution I and solution II were blended and emulsified, ultrasonicated for 10min, and put into an eggplant-shaped bottle after forming a stable emulsion , distilled under reduced...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com