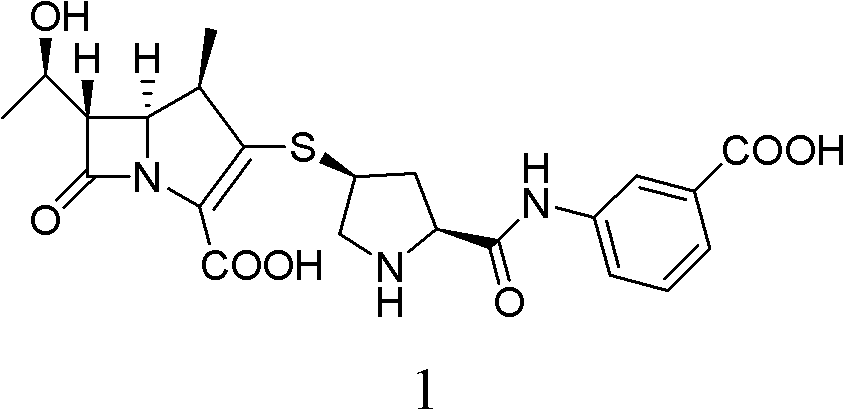
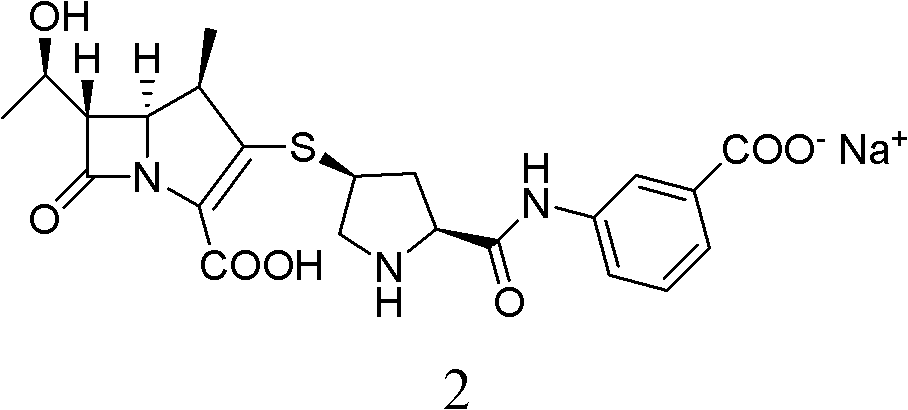
Method for preparing ertapenem sodium
A technology of ertapenem sodium and ertapenem, which is applied in the field of preparing carbapenem antibiotics, can solve problems such as low purity, and achieve the effects of improving purity, reducing cost and simplifying operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
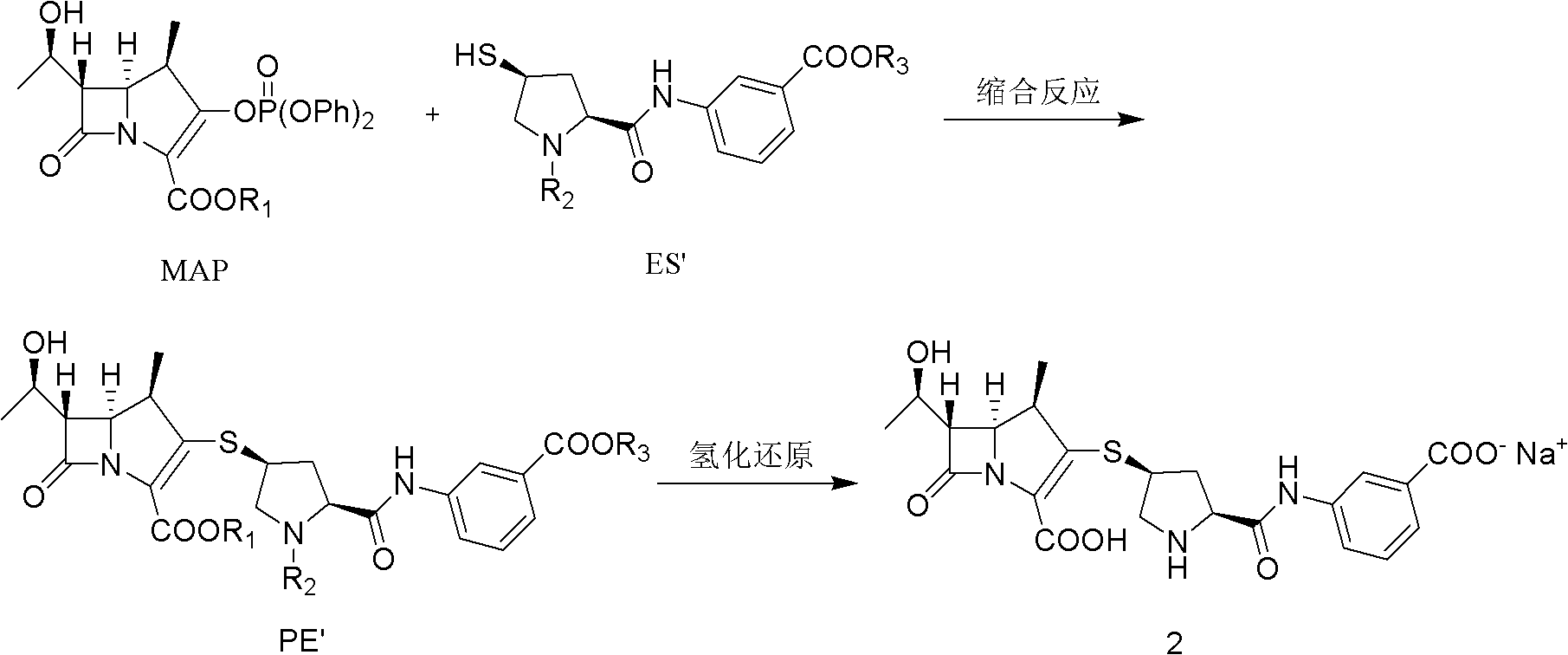
[0044] The ertapenem intermediate with a single protection structure is prepared by reacting 1β-methyl carbapenem bicyclic nucleus MAP with ertapenem side chain ES, and the specific operation steps are as follows:
[0045] A. Pass N through the 500ml four-necked bottle first 2 , add 100ml dimethylformamide (DMF), cool down to 0°C, then add 11.6g MAP, then add 6.48g side chain ES, add dropwise 0.3g tri-n-butylphosphine, stir to dissolve completely, then cool down to -50 °C, 8.1 g of tetramethylguanidine (TMG) was added dropwise, and 0.35 g of 4-N,N-dimethylaminopyridine (DMAP) was added, and the system temperature was maintained at -50 °C to -40 °C for 3 h.
[0046] B. Add 20ml saturated KH dropwise 2 PO 4 Aqueous solution, pH 6.5-7.5, stirred at -40°C for 30 minutes, then heated to 10°C.
[0047] C. Slowly add the solution obtained in step B into 1000ml of 1% hydrochloric acid solution dropwise, so that the pH of the solution is 5.5-5.9, stir for 30 minutes after the additi...
Embodiment 2
[0049] Ertapenem sodium is prepared by catalytically hydrogenating the ertapenem intermediate PE with a single protection structure, and the specific operation steps are as follows:
[0050] A. Add 7g of palladium carbon (7.5%) into 80ml of pure water and stir evenly.
[0051] B. the non-dried PE wet product (water content 65%) that embodiment 1 obtains is dissolved in 210ml tetrahydrofuran (THF), after completely dissolving, joins 60ml water (containing 5g NaHCO 3 , and the MAP molar ratio is 3 equivalents).
[0052] C. Add the solution obtained in step B to the solution obtained in step A, and hydrogenate for 90 minutes at 15-20° C. under 1.7 MPa.
[0053] D. Post-treatment: Pour out the hydrogenation solution, adjust the pH to 5.8 with 20% hydrochloric acid, filter with suction, and wash the filtrate with 300ml of cold CH 2 Cl 2 Extract once, separate the organic phase, filter the water layer, add 3g of activated carbon, stir for 10min, and filter with suction.
[0054]...
Embodiment 3
[0056] The ertapenem intermediate with a single protection structure is prepared by reacting 1β-methyl carbapenem bicyclic nucleus MAP with ertapenem side chain ES, and the specific operation steps are as follows:
[0057] A. Pass N through the 500ml four-necked bottle first 2 , add 100ml of dimethylformamide (DMF), cool down to 0°C, then add 11.6g of MAP, then add 6.48g of side chain ES, add 0.3g of tri-n-butylphosphine, stir to completely dissolve, then cool down to -50°C , add 7.9g of 4-N,N-dimethylaminopyridine (DMAP), keep the system temperature at -50°C to -40°C, and react for 3h.
[0058] B. Add 1.2g of glacial acetic acid dropwise, add 20ml of water, the pH is 6.5-7.5, stir at -40°C for 30min, and heat up to 10°C.
[0059] C. Slowly add the solution obtained in step B into 1000ml 3% KH 2 PO 4 In the aqueous solution, make the pH of the solution 5.5-5.9, stir for 30 minutes after the addition, filter with suction, and wash twice with ice water to obtain 30 g of PE we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com