C-glucoside derivative containing cyclopropane structure and method and application of C- glucoside derivative
A C1-C3, alkyl technology, applied in the field of C-glucoside type 2 sodium-glucose co-transporter inhibitor and its preparation, can solve the problems of hypoglycemia, weight gain and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1-{4-Chloro-3-[1-(4-ethoxyphenyl)cyclopropan-1-yl]phenyl}-1-deoxy-β-D-glucopyranose
[0046]
[0047] A. (5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone
[0048] Add 4.71g (20mmol) of 5-bromo-2-chlorobenzoic acid, 20mL of dry dichloromethane, 3.81g (30mmol) of redistilled oxalyl chloride and a drop of DMF to a 100mL dry round bottom flask to obtain a white cloudy mixture Stir overnight at room temperature until the system no longer gasses and becomes a clear solution. Evaporate excess oxalyl chloride and solvent on a rotary evaporator, dissolve the resulting residue with 15mL of dry dichloromethane, add 2.44g (20mmol) phenetole, stir under ice-water bath cooling, add 4.00g (30mmol) Anhydrous aluminum trichloride. After the addition was complete, the resulting mixture was stirred overnight at room temperature.
[0049] The reaction mixture was carefully poured into 200 mL of ice water, stirred, and extracted with 50 mL×3 of dichloromethane. The extract phases were...
Embodiment 2-13
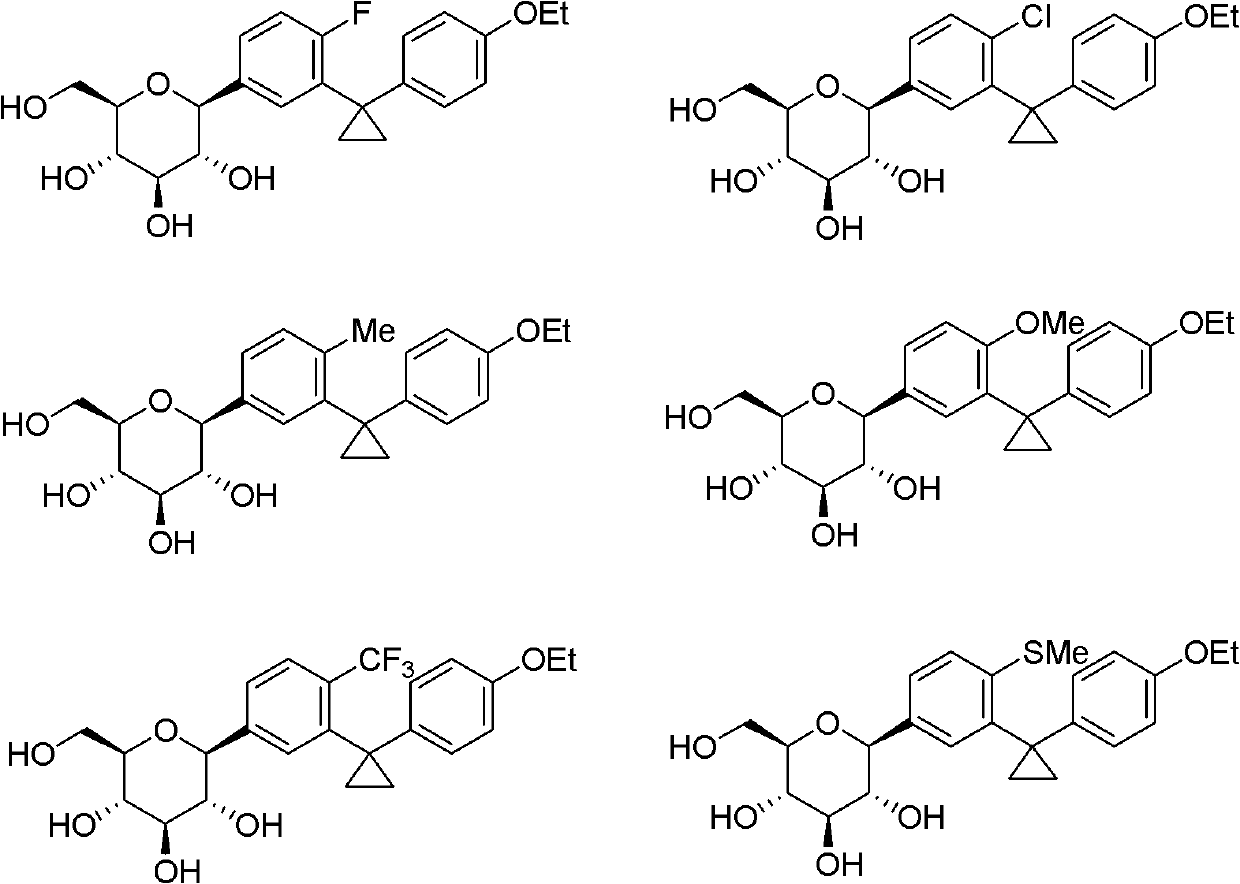
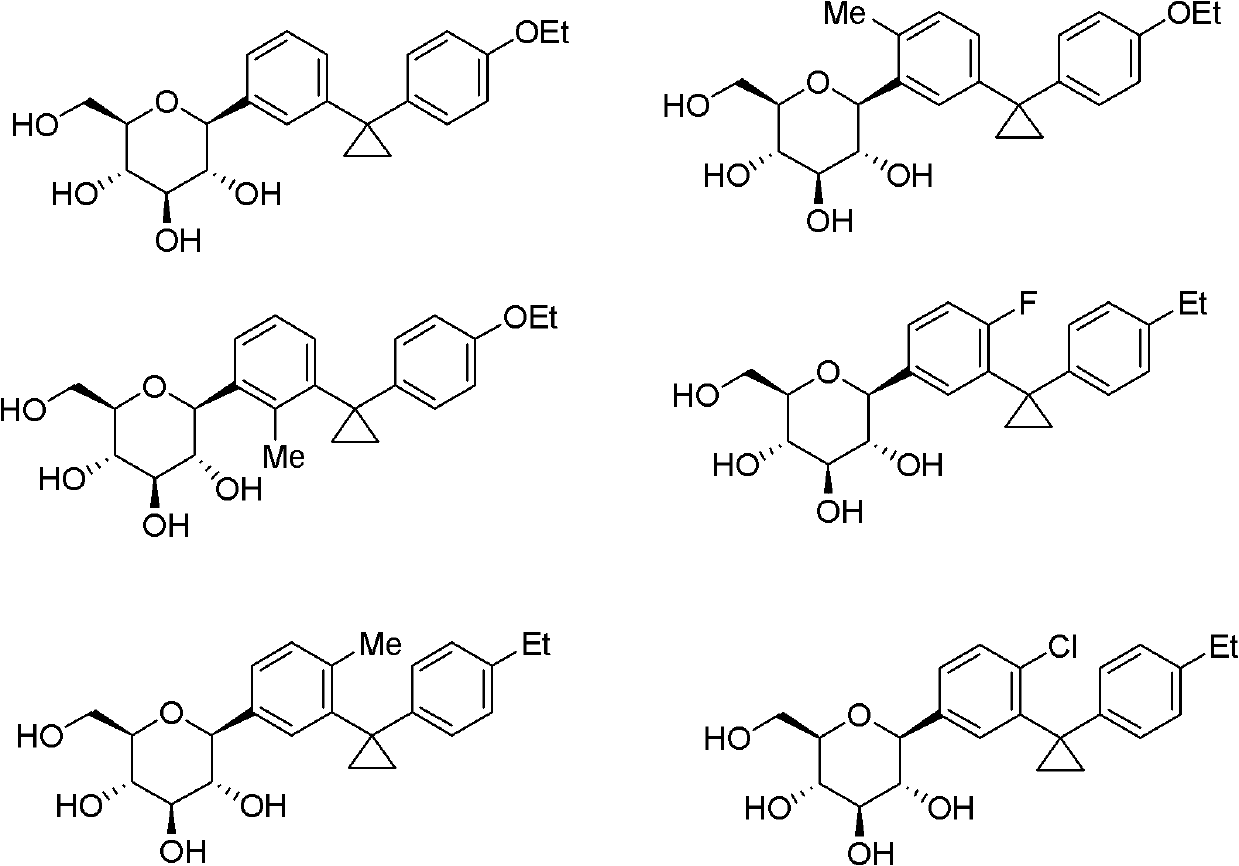
[0067] It can be understood that, using the method and process of Example 1, changing R 1 , R 2 The compounds listed in the table below can be obtained.
[0068]
[0069]
[0070]
Embodiment 14
[0072]
[0073]Sieve the active ingredient, pregelatinized starch and microcrystalline cellulose, mix well, add polyvinylpyrrolidone solution, mix, make soft material, sieve, make wet granules, dry at 50-60°C, and carboxymethyl starch Sodium salt, magnesium stearate and talc are pre-screened and then added to the above granules for tableting.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com