Preparation method of moxifloxacin hydrochloride
A technology of moxifloxacin hydrochloride and organic acid is applied in the field of preparation of moxifloxacin hydrochloride, and can solve the problems of difficulty in product purification, increased synthesis steps, increased types and quantities of reagents, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
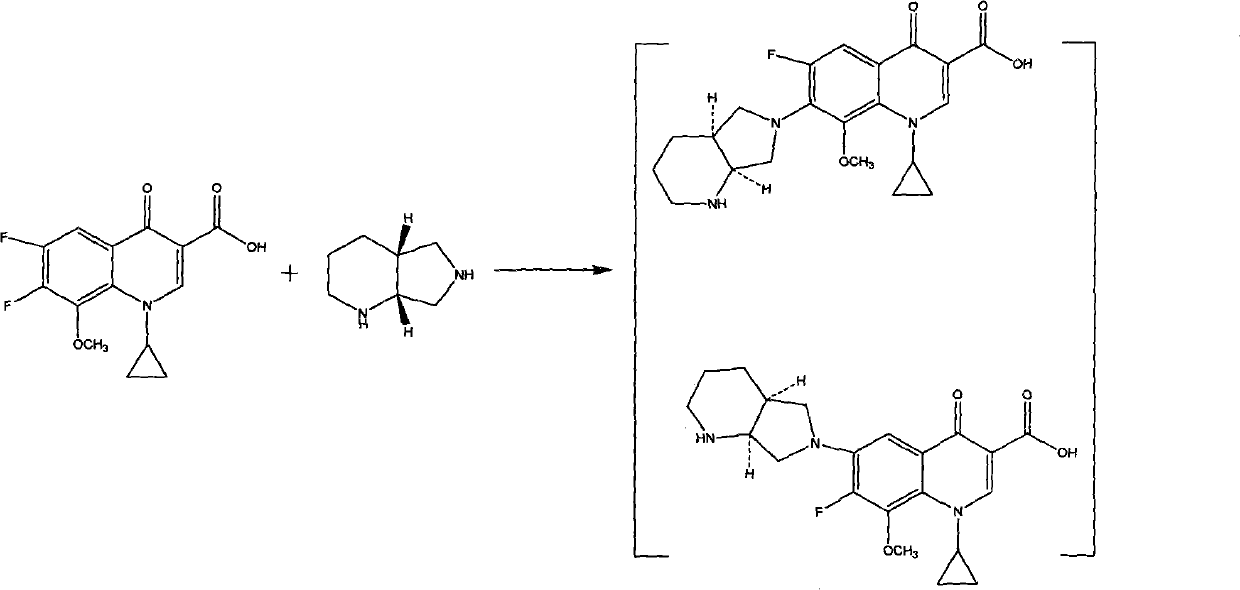
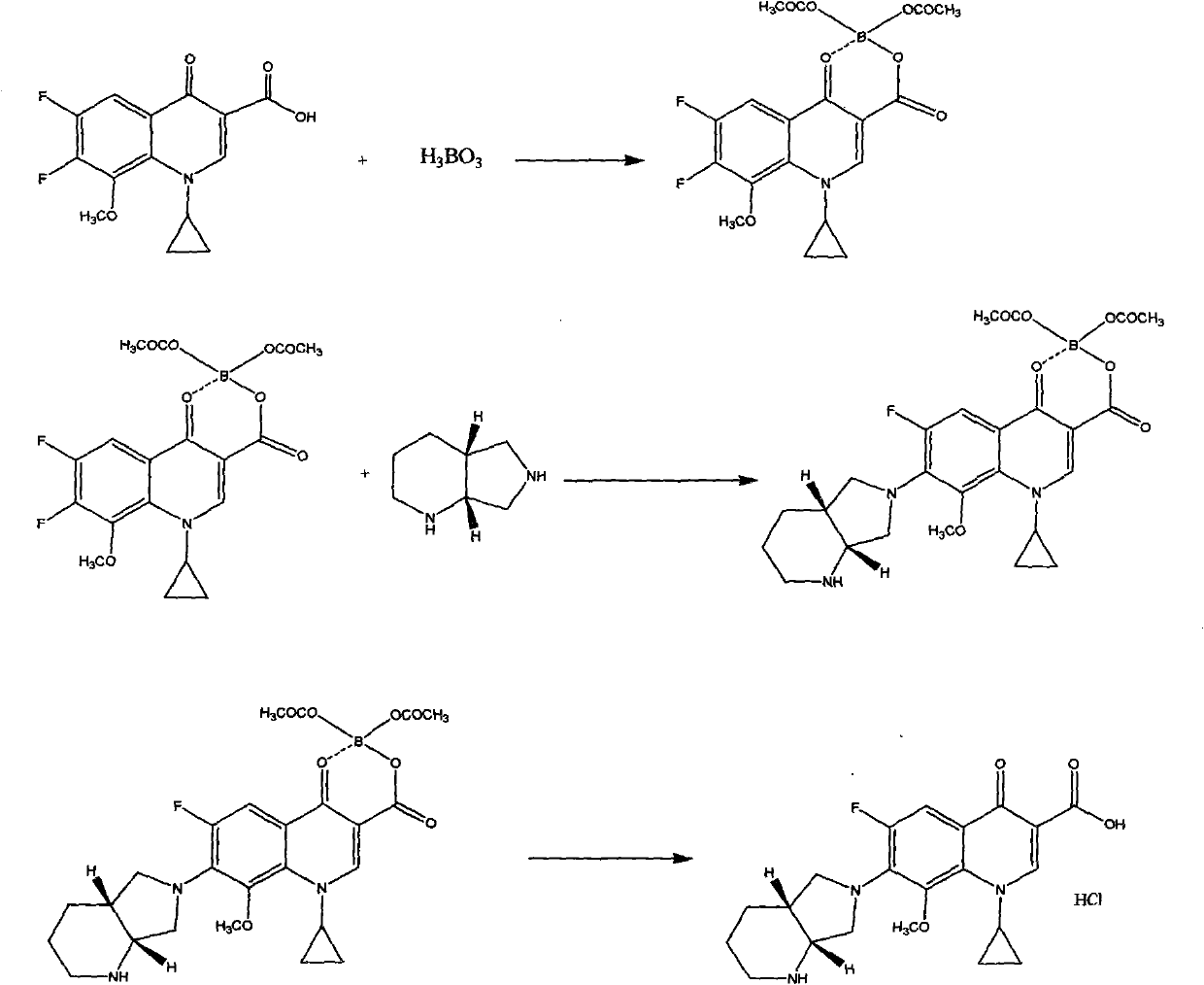
[0030] 1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl 】-The preparation of 4-oxo-3-quinoline carboxylic acid: add 50 grams (0.182 moles) 1-cyclopropyl-6,7-difluoro-8-methoxy-4 in the there-necked flask of 1000 milliliters -Oxo-1,4-dihydro-3-quinolinecarboxylic acid, 500 ml of N,N-dimethylformamide, dissolved under stirring, the above solution was cooled to 10 degrees Celsius, and 26 ml (0.204 mole) (S, S)-2,8-diazabicyclo【4.3.0】nonane, after adding, a large amount of solids were precipitated, under stirring, 18 grams (0.182 moles) of triethylamine was added and heated to 50 Celsius, and stirred at 40-50 Celsius for 8 hours, followed by thin-plate chromatography. After the reaction, add 100 ml of water and stir for 2 hours, filter, wash the filter cake with a small amount of water, and vacuum-dry at 70 Celsius to obtain 64 grams of solid powder .
Embodiment 2
[0032]1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl 】-The preparation of 4-oxo-3-quinoline carboxylic acid: add 50 grams (0.182 moles) 1-cyclopropyl-6,7-difluoro-8-methoxy-4 in the there-necked flask of 1000 milliliters -Oxo-1,4-dihydro-3-quinolinecarboxylic acid, 500 milliliters of dimethyl sulfoxide, dissolved under stirring, the above solution was cooled to 10 degrees Celsius, and 26 milliliters (0.204 moles) (S , S)-2,8-diazabicyclo【4.3.0】nonane, after the addition was completed, a large amount of solids were precipitated, under stirring, 14 grams (0.182 moles) of pyridine was added, heated to 50 degrees Celsius, and at 50- Stir at 60 degrees Celsius for 8 hours, thin-plate chromatography followed the reaction, after the reaction, add 100 milliliters of water and stir for 2 hours, filter, the filter cake is washed with a small amount of water, and vacuum-dried at 70 degrees Celsius to obtain 66 grams of solid powder.
Embodiment 3
[0034] 1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl 】-The preparation of 4-oxo-3-quinoline carboxylic acid: add 50 grams (0.182 moles) 1-cyclopropyl-6,7-difluoro-8-methoxy-4 in the there-necked flask of 1000 milliliters -Oxo-1,4-dihydro-3-quinolinecarboxylic acid, 500 ml of N,N-dimethylformamide and dimethyl sulfoxide mixed solvent (volume ratio is 1:1), dissolved under stirring , the above solution was cooled to 10 degrees Celsius, and 26 milliliters (0.204 moles) of (S, S)-2,8-diazabicyclo[4.3.0]nonane were added under constant stirring. After adding, a large amount of solids were precipitated. Under stirring, add 23.5 grams (0.182 moles) of diisopropylethylamine, heat to 50 degrees Celsius, and stir at 55-65 degrees Celsius for 8 hours, thin-plate chromatography follows the reaction, after the reaction finishes, add 100 milliliters of water and stir for 2 hour, filtered, and the filter cake was washed with a small amount ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com