Novel tricyclic protein kinase modulators
A solvate and compound technology, applied in the field of new tricyclic protein kinase regulators, can solve problems such as dependence and MM cell cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
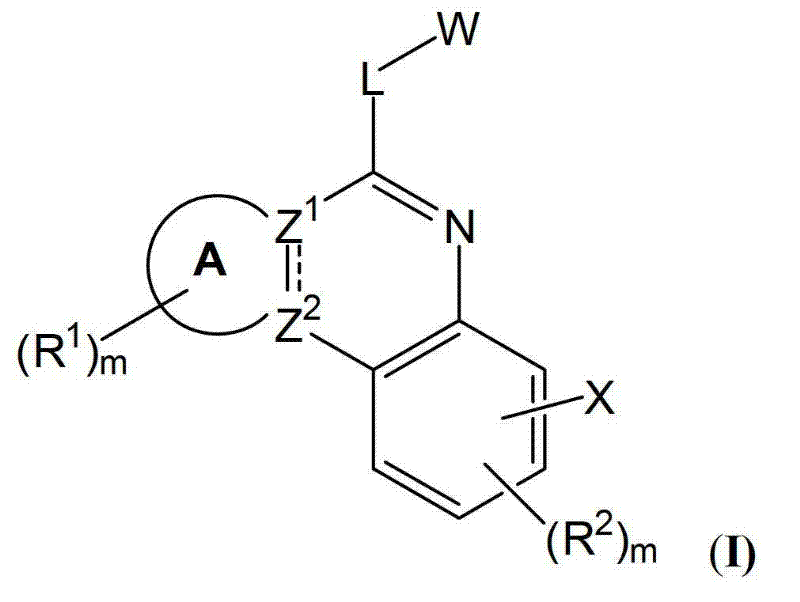
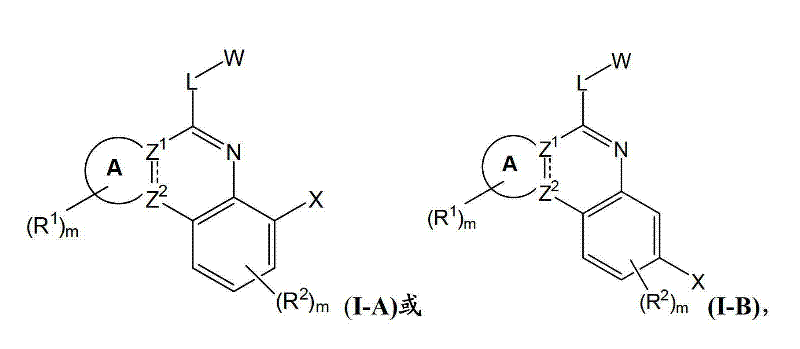
[0163] In one aspect, the present invention provides a compound of formula I or a pharmaceutically acceptable salt, solvate and / or prodrug thereof:
[0164]
[0165] in:
[0166] A is an optionally substituted saturated or partially saturated 5, 6 or 7 membered ring;
[0167] Indicates a single or double bond;
[0168] when When representing a single key, Z 1 and Z 2 independently N or C with the proviso that Z 1 and Z 2 not all N; and
[0169] when When representing a double bond, Z 1 and Z 2 is C;
[0170] L is a linker selected from bond, NR 3 , O, S, CR 4 R 5 、CR 4 R 5 -NR 3 、CR 4 R 5 -O-, and CR 4 R 5 -S;
[0171] R 1 , R 2 , R 3 , R 4 and R 5 each independently is H, or an optionally substituted member selected from the group consisting of C1-C8 alkyl, C2-C8 heteroalkyl, C2-C8 alkenyl, C2-C8 heteroalkenyl, C2-C8 alkyne Group, C2-C8 heteroalkynyl, C1-C8 acyl, C2-C8 heteroacyl, C6-C10 aryl, C5-C12 heteroaryl, C7-C12 aralkyl and C6-C12 hete...
Embodiment 1
[0349]The chemistry described in Scheme 1 can be used to prepare intermediate 4 with a tetrahydrothiopyran ring. Compound 2 preparation was previously described in WO2009061131. Compound 3 can be formed using the method described in WO2009061131 by heating commercially available isocyanate 1 and compound 2 in toluene and by subsequent treatment of the reaction mixture with acid. Compound 3 can be converted to compound 4 using an acid such as sulfuric acid.
[0350] plan 1
[0351]
Embodiment 2
[0353] The chemistry described in Example 1 can be applied to other substituted isocyanates 2 (Scheme 2) to prepare analog 6 with various substitutions on the phenyl ring. Isocyanate 2 can be commercially available or prepared from commercially available aniline 1 .
[0354] Scenario 2
[0355]
[0356] The chemistry can also be applied to substituted 2-bromoanilines 4 to obtain compounds 6. Compound 6 can be converted to compound 3 in two steps by treatment with a cyanide reagent, followed by hydrolysis and esterification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com