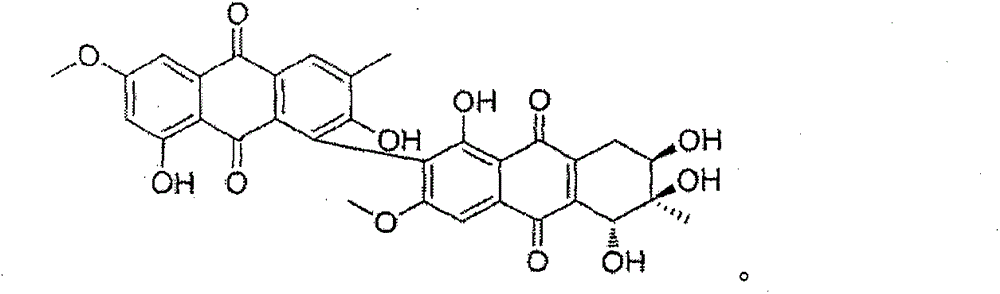
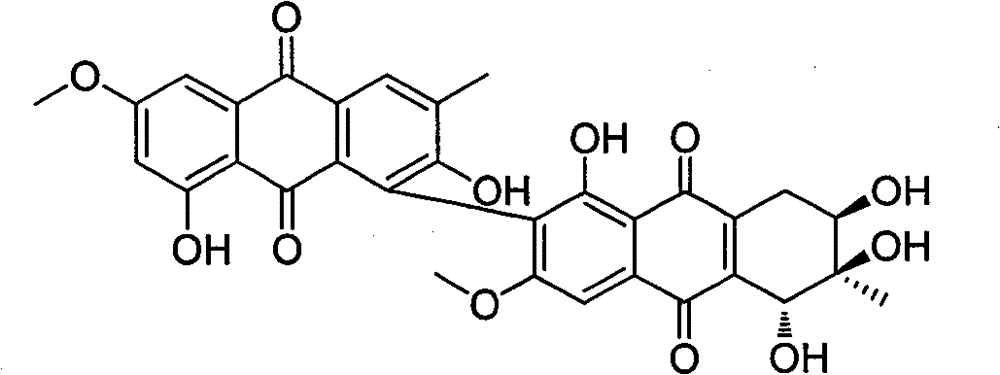
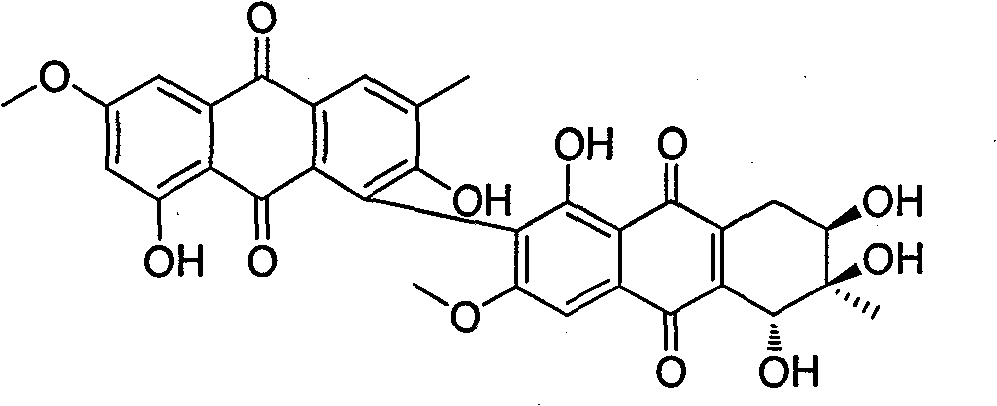
Anthraquinone dimer derivative alterporriol P and its preparation method and application
An anthraquinone dimer and derivative technology, which is applied to the anthraquinone dimer derivative Alterporriol P and its preparation and application fields, can solve problems such as no anti-tumor drugs, and achieve obvious proliferation inhibitory activity and application prospects. expansive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0009] In preparing the compounds of the present invention, the following steps are taken:
[0010] (1) Carry out strain culture to the endophytic fungus Alternariasp. (ZJ-2008003) that is derived from the soft coral Sarcophytonsp. in the South China Sea earlier in the strain culture medium, and the strain culture medium used contains glucose 2.0% (percentage by weight, The same below), potato 20.0%, agar 1.5%, coarse sea salt 3.0%, and the rest is water, which is made into a test tube slant during use, and the above-mentioned fungal strains are cultivated at 28° C. for 5 days.
[0011] The strain culture medium contains 0.1%-5.0% of glucose (percentage by weight, the same below), 5.0%-40.0% of potato, 0.1%-3.0% of agar, 0.05%-5.0% of coarse sea salt, and the rest is water. The temperature is 15-35°C, and the cultivation time is 3-10 days.
[0012] (2) The fungal strains obtained from the above culture are fermented, and the fermentation medium used contains 2.0% glucose, 20....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com