Indole-3-carboxaldehyde isobutyryl hydrazone derivatives and preparation method thereof
A technology of indole derivatives and compounds, which is applied in the field of indole-3-formaldehyde isobutyryl hydrazone derivatives and its preparation, and can solve problems such as tumor recurrence, drug insensitivity, and tumor cells prone to drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
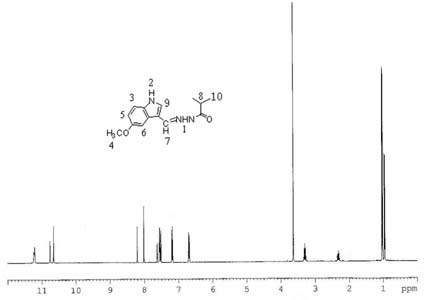
[0025] Add 0.88g of 5-methoxyindole-3-carbaldehyde, 0.52g of isobutyrhydrazide and 30ml of anhydrous methanol into a 100ml three-necked flask, slowly raise the temperature to 60°C under stirring, keep the temperature for 12h, filter with suction, and filter cake After drying, it was recrystallized with acetone to obtain white crystals, and compound 1 was obtained after vacuum drying with a yield of 63.81%. MS (ESP) m / z: 259 (M);
[0026] Elemental analysis (C 14 h 17 N 3 o 2 ): %C: 64.72 (64.85); %H: 6.49 (6.61); %N: 16.48 (16.20); %O: 12.57 (12.34) (calculated values in brackets)
[0027] NMR analysis (NMR spectrum see figure 1 ):
[0028] atomic number 1 2 3 4 5 6 7 8 9 10 δ / 1 H, ppm 11.22 10.66 7.52 3.64 6.70 7.18 8.20 3.18 7.56 1.01 Proton number 1 1 1 3 1 1 1 1 1 6 split fraction d d d s m m d m m m
[0029] s: singlet; d: doublet; m: multiplet.
Embodiment 2
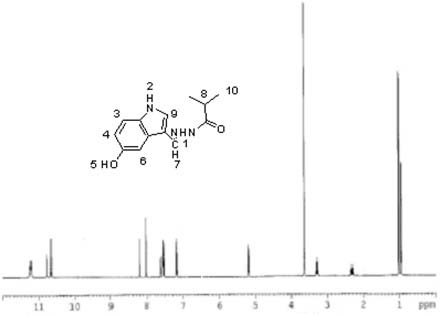
[0031] Add 1.61g of 5-oxindole-3-carbaldehyde, 1.38g of isobutyrhydrazide and 70ml of absolute ethanol into a 100ml three-necked flask, slowly heat up to 50°C under stirring, keep the temperature for 9 hours, filter with suction, and dry the filter cake White crystals were obtained by recrystallization from ethyl acetate, and compound 2 was obtained after vacuum drying with a yield of 63.06%. MS (ESP) m / z: 245 (M);
[0032] Elemental analysis (C 13 h 15 N 3 o 2 ): %C: 63.52 (63.66); %H: 6.09 (6.16); %N: 17.38 (17.13); %O: 13.19 (13.05) (calculated values in brackets)
[0033] NMR analysis (NMR spectrum see figure 2 ):
[0034] atomic number 1 2 3 4 5 6 7 8 9 10 δ / 1 H, ppm 11.22 10.66 7.52 3.64 5.25 7.18 8.20 3.18 7.56 1.01 Proton number 1 1 1 3 1 1 1 1 1 6 split fraction d d d s m m d m m m
[0035] s: singlet; d: doublet; m: multiplet.
Embodiment 3
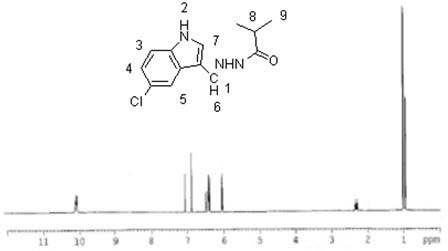
[0037] Add 1.79g of 5-chloroindole-3-carbaldehyde, 1.85g of isobutyrhydrazide and 30ml of anhydrous methanol into a 100ml three-necked flask, slowly raise the temperature to 45°C under stirring, keep the temperature for 12h, filter with suction, and dry the filter cake White crystals were obtained by recrystallization from petroleum ether, and compound 3 was obtained after vacuum drying with a yield of 61.75%. MS (ESP) m / z: 263 (M);
[0038] Elemental analysis (C 13 h 14 ClN 3O): %C: 59.48 (59.21); %H: 5.21 (5.35); %Cl: 13.56 (13.44); %N: 15.81 (15.93); %O: 6.14 (6.07) (calculated values in brackets)
[0039] NMR analysis (NMR spectrum see image 3 ):
[0040] atomic number 1 2 3 4 5 6 7 8 9 δ / 1 H, ppm 7.00 10.10 7.30 7.10 7.60 7.50 7.30 2.78 1.19 Proton number 1 1 1 1 1 1 1 1 6 split fraction d d d s s s d m m
[0041] s: singlet; d: doublet; m: multiplet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com