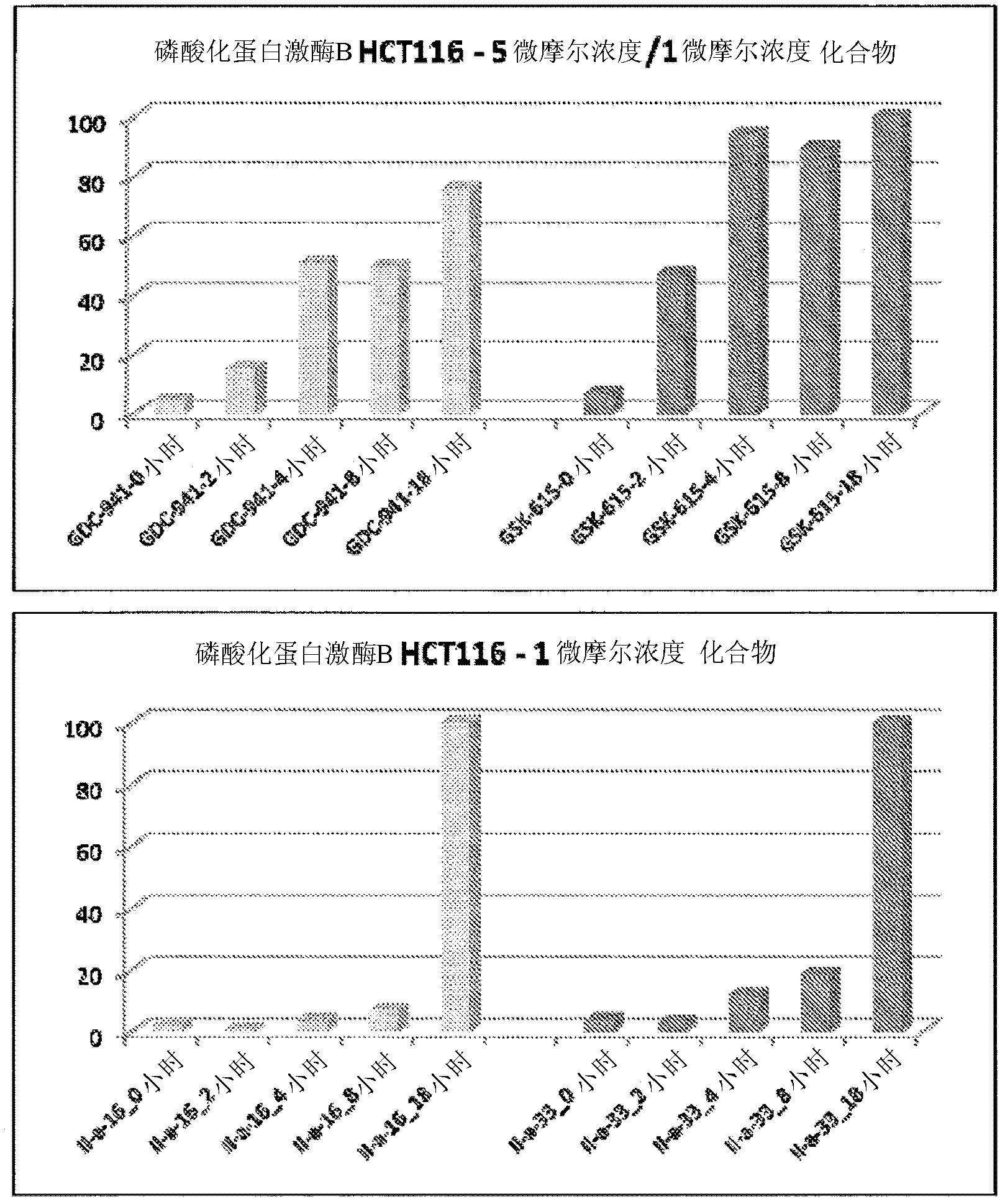
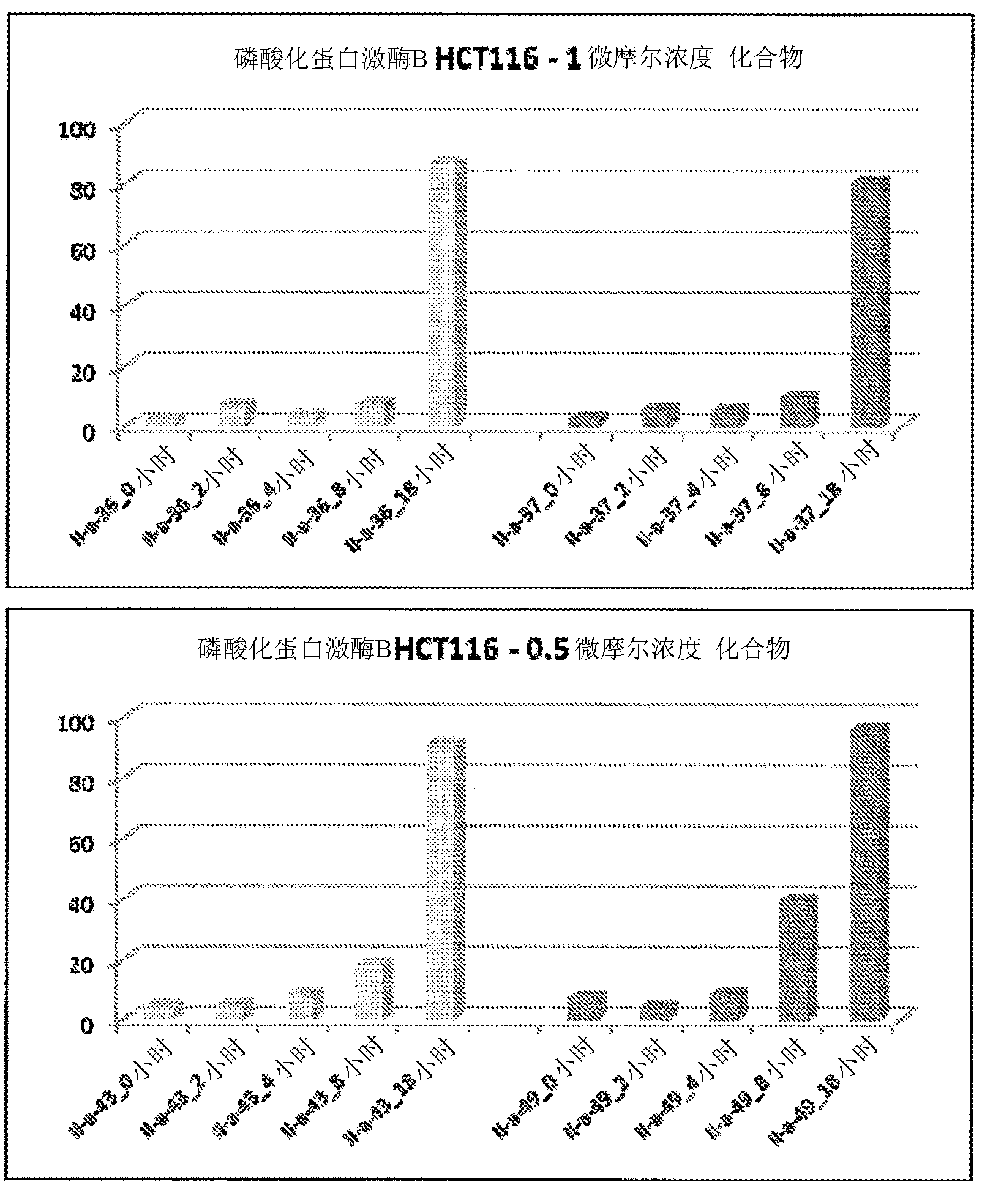
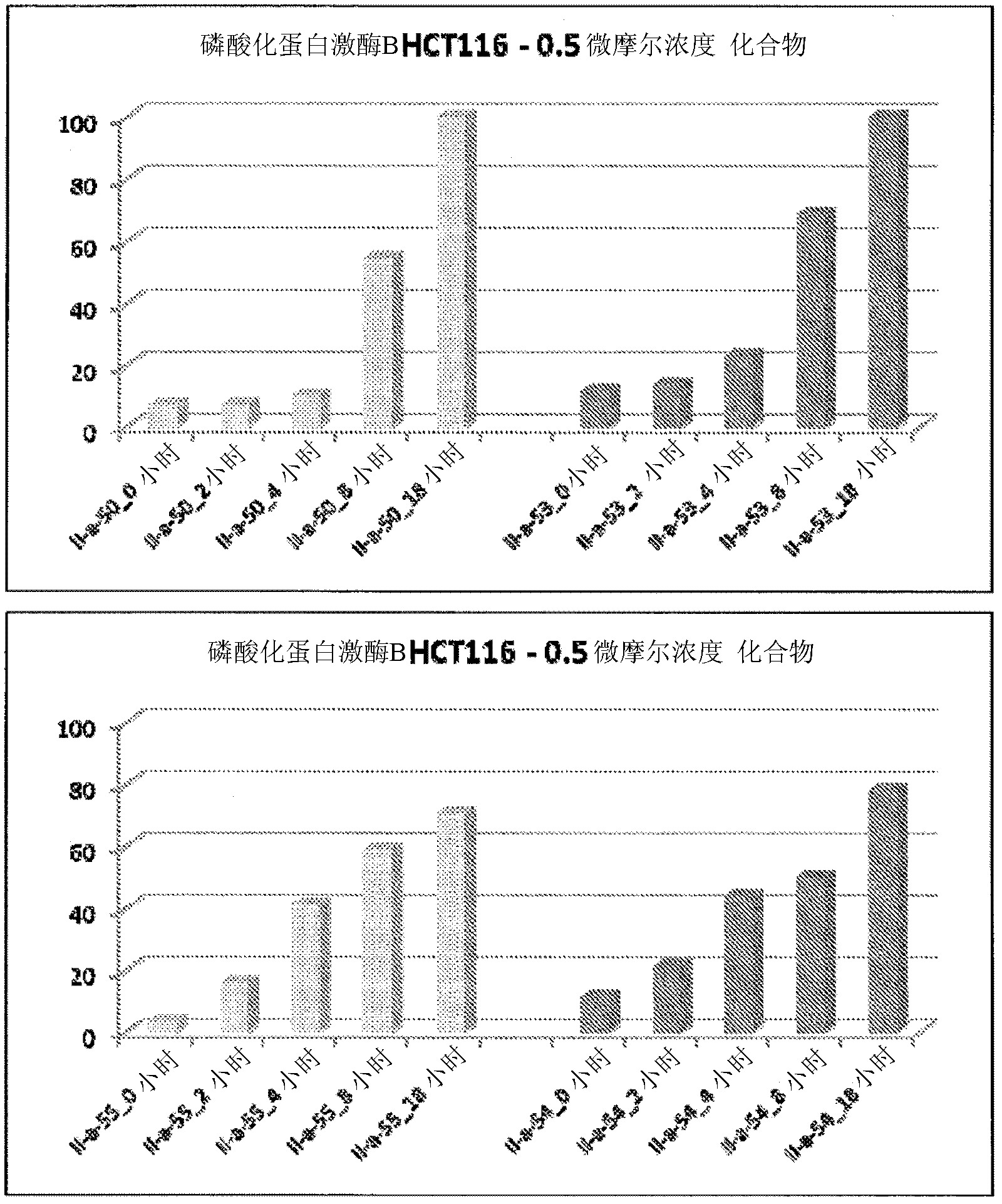
PI3 kinase inhibitors and uses thereof
A technology of inhibitors and kinases, applied in the direction of enzymes, anti-inflammatory agents, antiviral agents, etc., can solve the problem that the regulatory subunit of class II PI3K has not been identified
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[1378]
[1379] 1-(4-((2-(1H-indazol-4-yl)-4-(N-morpholino)thieno[3,2-d]pyrimidin-6-yl)methyl)piperazine- 1-yl)prop-2-en-1-one (II-a-2): The title compound was prepared according to the procedures and intermediates described below.
[1380]
[1381] Step 1a: 4-(2-Chlorothieno[3,2-d]pyrimidin-4-yl)morpholine (intermediate 1a)
[1382]
[1383] To a solution of 2,4-dichlorothieno[3,2-d]pyrimidine (2.0 g, 9.7 mmol) in 30 ml MeOH was added 1.9 ml morpholine. After stirring at room temperature for 1 hour, the reaction mixture was filtered; the solid was washed with water and methanol to afford 2.0 g of the title compound. MS m / z: 256.0, 258.1 (M+1). 1 H NMR (400MHz, CDCl 3 ): δ: 7.78 (1H, d, J = 5.48Hz), 7.38 (1H, d, J = 5.48Hz), 4.02 (4H, t, J = 4.80Hz), 3.85 (4H, t, J = 4.82Hz ).
[1384] Step 1b: 2-Chloro-4-(N-morpholino)thieno[3,2-d]pyrimidine-6-carbaldehyde (intermediate 1b)
[1385]
[1386] To a suspension of intermediate 1a (1.02 g, 4.0 mmol) in 30 ml THF ...
example 2
[1416]
[1417] (E)-1-(4-((2-(1H-indazol-4-yl)-4-(N-morpholinyl)thieno[3,2-d]pyrimidin-6-yl)methyl )piperazin-1-yl)hept-5-ene-1,4-dione (II-a-36): The title compound was prepared according to the procedures and intermediates described below.
[1418] Step 2a: (E)-4-oxohept-5-enoic acid (intermediate 2a)
[1419]
[1420] To a solution of succinic anhydride (0.50 g, 5.0 mmol) in 20.0 ml dry THF was slowly added 1-propenylmagnesium bromide (0.5 M in THF, 18.0 mL, 9.0 mmol) at -78°C. The reaction mixture was stirred at -78°C for 1 hour. 1N Aqueous HCl (9.0 ml) was added and the mixture was slowly warmed to room temperature. The pH was adjusted to about 3 with 1N HCl. Then THF was removed under vacuum and the remaining aqueous solution was extracted with DCM (3 x 20 mL). The organic layer was washed with Na 2 SO 4 Dry, filter and remove solvent. The residue was purified by silica gel chromatography (eluent: EtOAc / Hexane 1:1) to afford the acid. 1 H NMR (400MHz, CDCl ...
example 3
[1462]
[1463] N-(2-(4-((2-(1H-indazol-4-yl)-4-(N-morpholinyl)thieno[3,2-d]pyrimidin-6-yl)methyl) Piperazin-1-yl)-2-oxoethyl)acrylamide (II-a-6): The title compound was prepared according to the procedures and intermediates described below.
[1464] Step 3a: 2-(4-((2-(1H-Indazol-4-yl)-4-(N-morpholinyl)thieno[3,2-d]pyrimidin-6-yl)methyl) tert-butyl piperazin-1-yl)-2-oxoethylcarbamate (intermediate 3a)
[1465]
[1466] The title compound was prepared by coupling BOC-Gly-OH with intermediate 1e using HATU according to the procedure described in step 1f. MS m / z: 593.2 (M+H + ).
[1467] Step 3b: 1-(4-((2-(1H-indazol-4-yl)-4-(N-morpholinyl)thieno[3,2-d]pyrimidin-6-yl)methyl) Piperazin-1-yl)-2-aminoethanone hydrochloride (intermediate 3b)
[1468]
[1469] The title compound was prepared by the BOC removal procedure described in Step 1e. MS m / z: 493.2 (M+H + ).
[1470] Step 3c: N-(2-(4-((2-(1H-indazol-4-yl)-4-(N-morpholinyl)thieno[3,2-d]pyrimidin-6-yl) Methyl)pi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com