3-nitro-8-ethyoxyl-2H-chromene compound and preparation method and application thereof
A technology of benzopyran and ethoxy, which is applied in the field of medicine, can solve the problems of drug resistance target specificity and broad spectrum, and achieve the effect of increasing the expression level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Preparation of 3-nitro-8-ethoxy-2H-benzopyran (YL201211)
[0045] 3-Ethoxy salicylaldehyde (16.6 g, 100 mmol) was dissolved in 800 ml of toluene, dibutylamine (12.9 g, 100 mmol), phthalic anhydride (29.6 g, 200 mmol) were added ), heated to reflux, nitroethanol (11.8 g, 130 mmol) was added 13 times within 12 hours, and the reaction was continued for 24 hours; the solvent was evaporated, and the compound YL201211 (14.2 g) was obtained by column chromatography.
Embodiment 2
[0046] Example 2: Preparation of 3-nitro-6-bromo-8-ethoxy-2H-benzopyran (YL201212)
[0047] 5-Bromo-3-ethoxy salicylaldehyde (24.5 g, 100 mmol) was dissolved in 800 ml of toluene, di-n-butylamine (12.9 g, 100 mmol), phthalic anhydride (29.6 g, 200 mmol), heated to reflux, added nitroethanol (11.8 g, 130 mmol) in 13 times within 12 hours, continued the reaction for 24 hours, evaporated the solvent, and obtained compound YL201212 (17.1 g) by column chromatography.
Embodiment 3
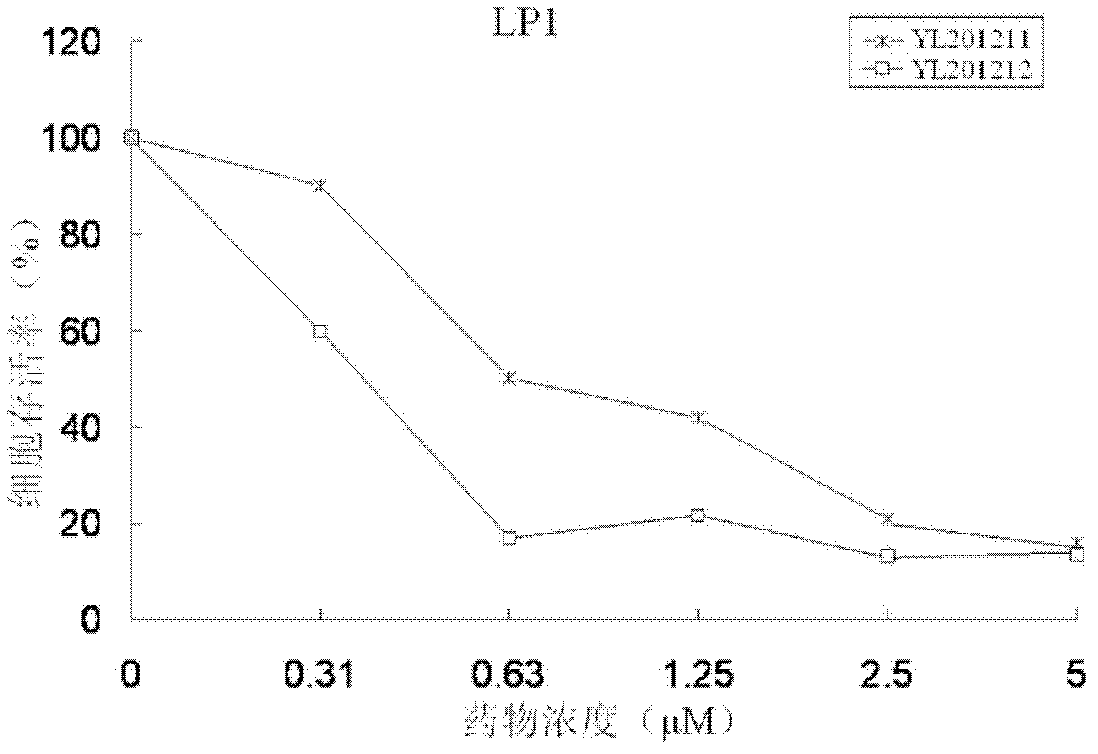
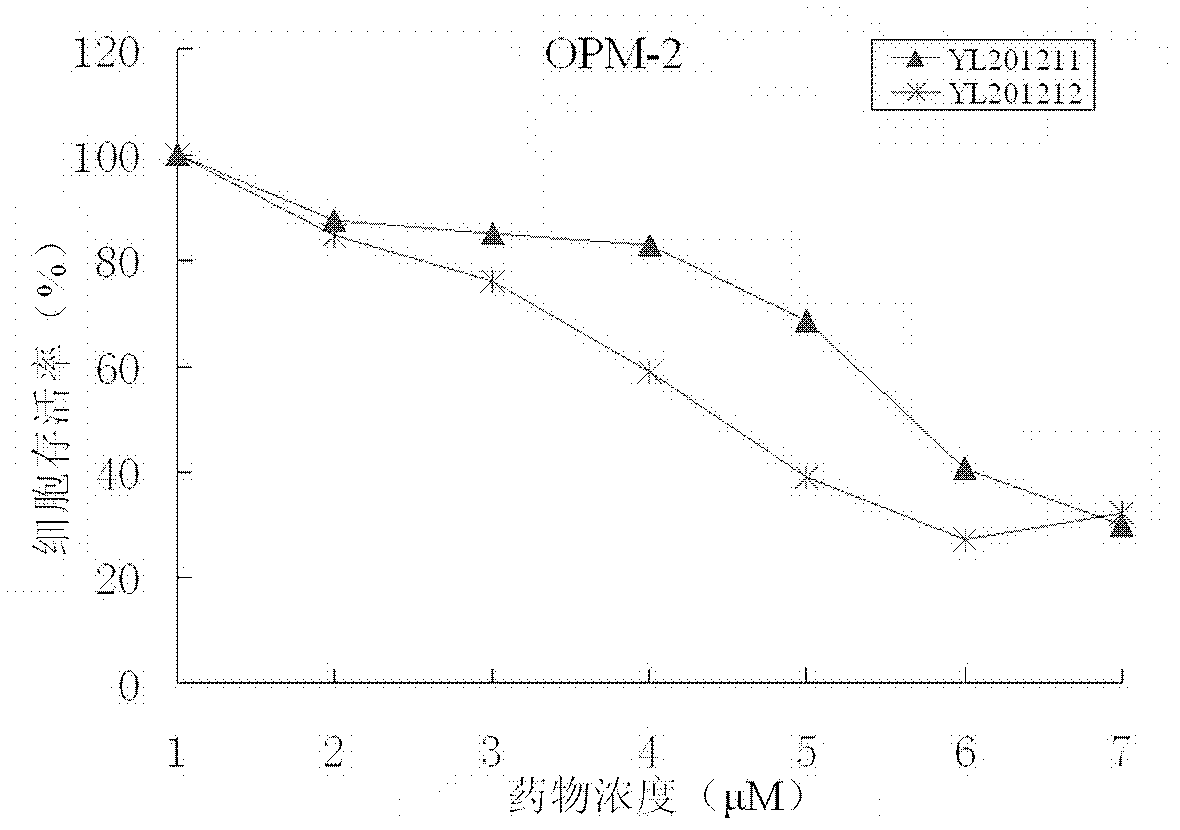
[0048] Example 3: Inhibition by 3-nitro-8-ethoxy-2H-benzopyran (YL201211) and 3-nitro-6-bromo-8-ethoxy-2H-benzopyran (YL201212) Proliferation assay of malignant myeloma and leukemia cells
[0049] Explanation of terms:
[0050] MTT: 3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide, trade name: thiazolium blue; DMSO: dimethylsulfoxide.
[0051] Test materials and methods:
[0052] Experimental materials: LP1 cells, OPM-2 cells, K562 cells, Jurkat cells, 96-well plate, MTT, 10% DMSO (volume ratio).
[0053] LP1 cells, OPM-2 cells, K562 cells, and Jurkat cells were all provided by Tang Zhongying Blood Center of Soochow University.
[0054] The specific operation is as follows:
[0055] The myeloma cell line LP1, the myeloma cell line OPM-2, the leukemia cell line K562, and the leukemia cell line Jurkat were evenly inoculated in a 96-well plate at a density of 10,000 cells / ml, and 100 microliters of cell. The cell culture environment is 37°C, 5% carbon dioxide (vol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com