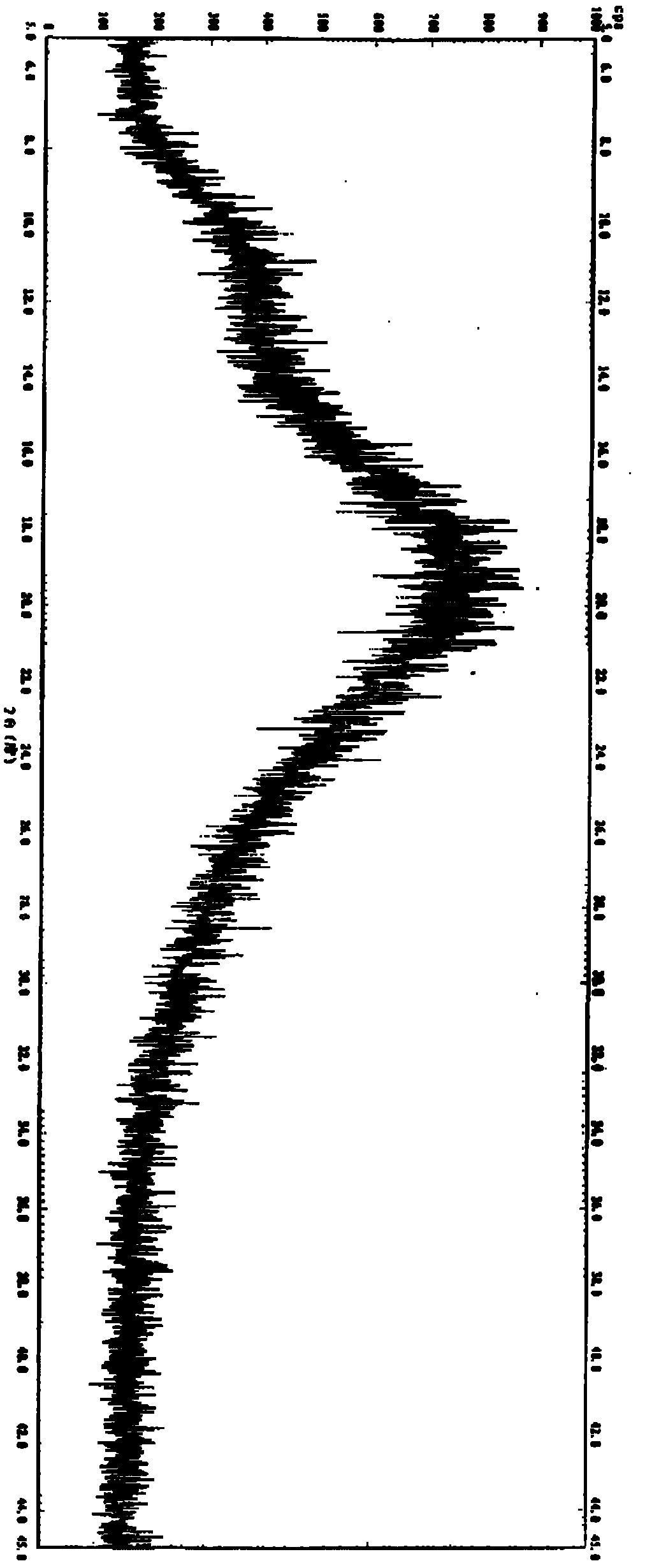
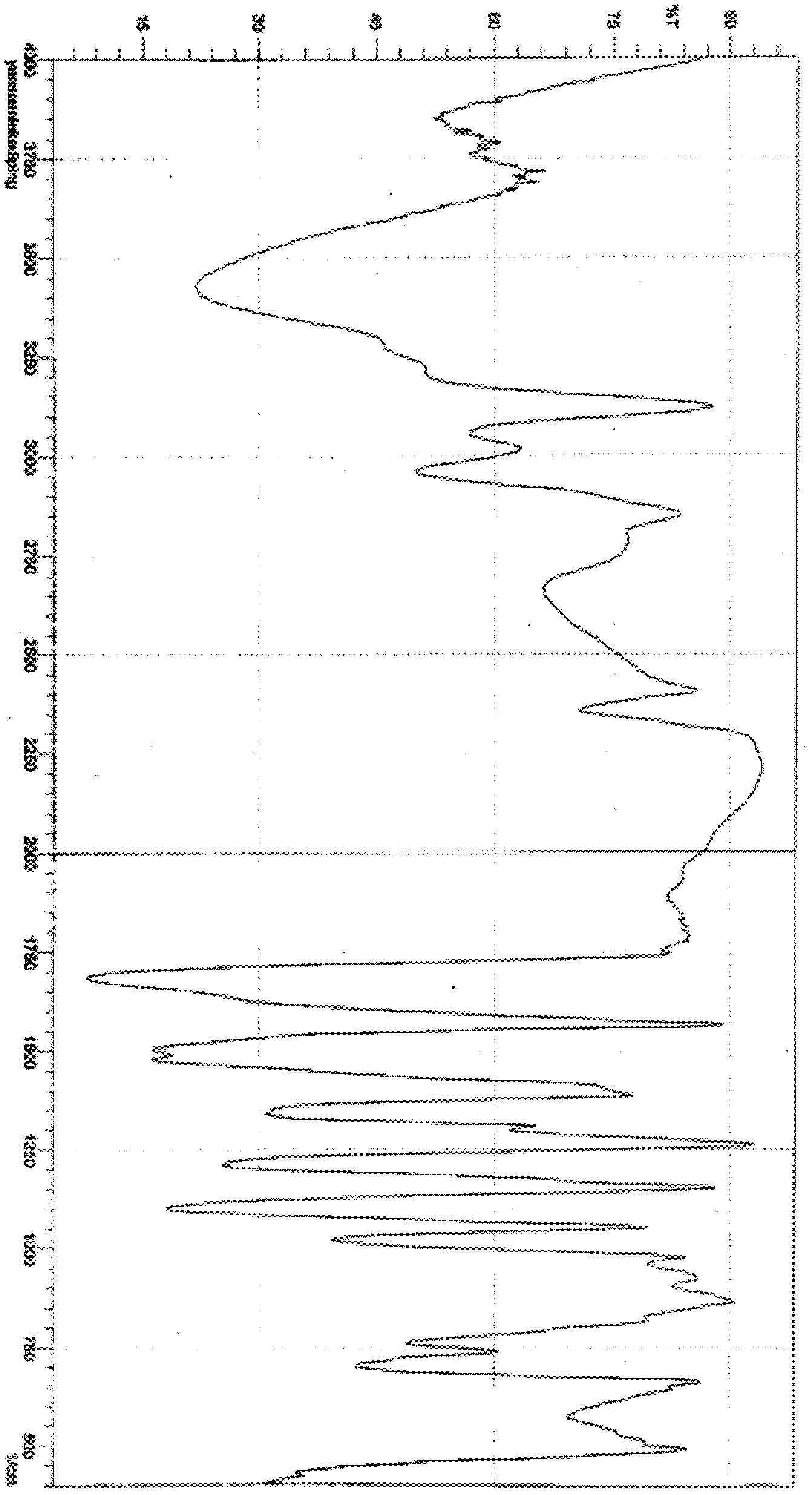
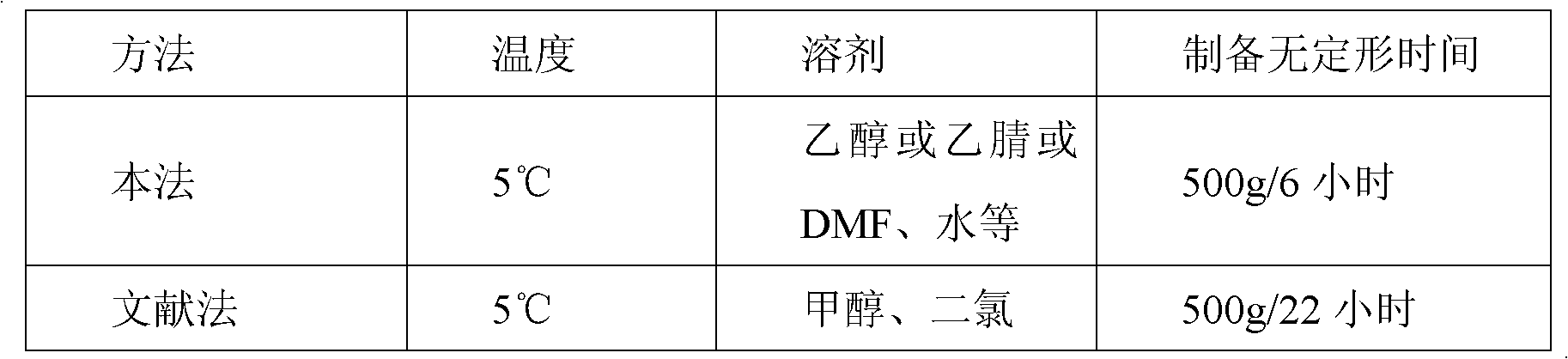
Amorphous lercanidipine hydrochloride and preparation method thereof
A lercanidipine hydrochloride, amorphous technology, used in pharmaceutical formulations, medical preparations containing active ingredients, cardiovascular system diseases, etc., can solve the problems of high cost, long cycle, cumbersome operation, etc., and achieve low cost and cycle time. Short, high-purity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Add 10g of high-purity lercanidipine hydrochloride into 100mL of ethylene glycol dimethyl ether, adjust the pH to 7.5-8.0 with saturated aqueous sodium bicarbonate solution at 5°C under stirring, leave to stand and separate the layers, and separate the organic layer. Dry over sodium sulfate, filter, evaporate the filtrate under reduced pressure to remove most of the solvent, and dissolve the light yellow oil in 30ml of isopropanol for later use.
Embodiment 2
[0039] The preparation of embodiment 2 high-purity free state lercanidipine base DMF solution
[0040] Add 10g of high-purity lercanidipine hydrochloride into 100mL of ethylene glycol dimethyl ether, adjust the pH to 7.5-8.0 with saturated aqueous sodium bicarbonate solution at 5°C under stirring, leave to stand and separate the layers, and separate the organic layer. Dry over sodium sulfate, filter, and evaporate the filtrate under reduced pressure to remove most of the solvent. The light yellow oil is dissolved in 20ml of DMF and set aside.
Embodiment 3
[0041] Embodiment 3 Preparation of high-purity free state lercanidipine base acetonitrile solution
[0042] Add 10g of high-purity lercanidipine hydrochloride into 100mL of ethylene glycol dimethyl ether, adjust the pH to 7.5-8.0 with saturated aqueous sodium bicarbonate solution at 5°C under stirring, leave to stand and separate the layers, and separate the organic layer. Dry over sodium sulfate, filter, evaporate the filtrate under reduced pressure to remove most of the solvent, and dissolve the light yellow oil in 20ml of acetonitrile for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com