Early diagnosis and early warning kit for transplanted kidney rejection reaction and detection method
A technology for rejection and early diagnosis, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as difficulty, difficulty, and complicated diagnosis of transplant rejection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1 Screening of Significant Difference Markers in Early Diagnosis Kit for Transplanted Kidney Rejection
[0105] 1. Collect serum of kidney transplant recipients at multiple time points after transplantation, and analyze and group clinical cases.
[0106] 1.1 Select kidney transplant recipients with preoperative basic diseases or few complications, and no serious complications during the operation, and select kidney transplant recipients at 1 day, 7 days, 14 days, 21 days, 28 days, and 2 months after transplantation. 1. Collect 2ml of whole blood from 3 months to 12 months, let stand at room temperature for 1 hour, centrifuge at 1000rpm for 10 minutes to collect the supernatant, store in -80°C refrigerator after aliquoting. In addition, the serum of the patients in the acute rejection group after the diagnosis of acute rejection was collected at any time.
[0107]1.2 According to the comprehensive analysis and case screening of serum creatinine, urea nitrogen, t...
Embodiment 2
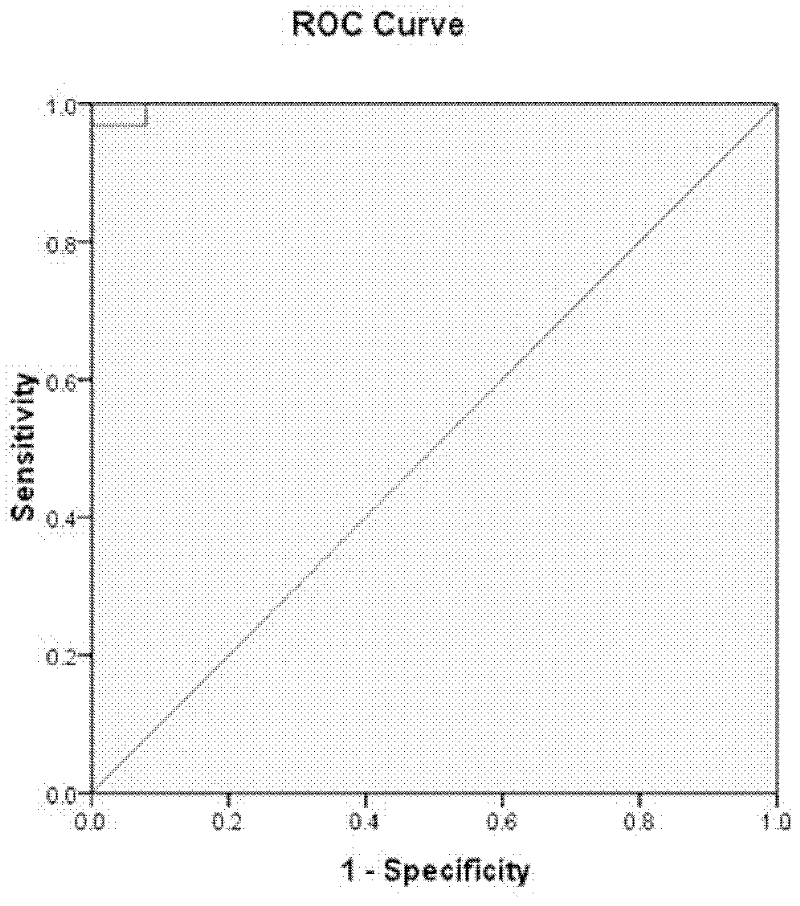
[0123] The 35 markers with significant differences obtained in Example 1 were analyzed, and the markers with significant differences in expression levels were analyzed by logistic regression to obtain 4 factors: SCF, sEGFR, sIL2Ralpha and Eotaxin. The early warning and diagnosis system of acute rejection, the statistical analysis shows that the predicted positive rate is 91.4. The area under the ROC diagnostic curve is 97.5%, see attached figure 1 .
[0124] Wherein, the concrete composition of kit is:
[0125] (1) 96-well filter plate and two sealing membranes; purchased from Millipore Company, item number MX-PLATE;
[0126] (2) Standards of protein antibodies in the kit; purchased from Millipore Company, catalog numbers MXH8060, MXH8062, MXH8063, LHSP-8063 and HSCR-8032;
[0127] (3) Quality control control; purchased from Millipore Company, the article numbers are: MXH6060, MXH6062, MXH6063, LHSP-6063 and HSCR-6032;
[0128] (4) Serum matrix; purchased from Millipore Co...
Embodiment 3
[0135] Example 3: The composition of the kit for early diagnosis of acute rejection of transplanted kidney:
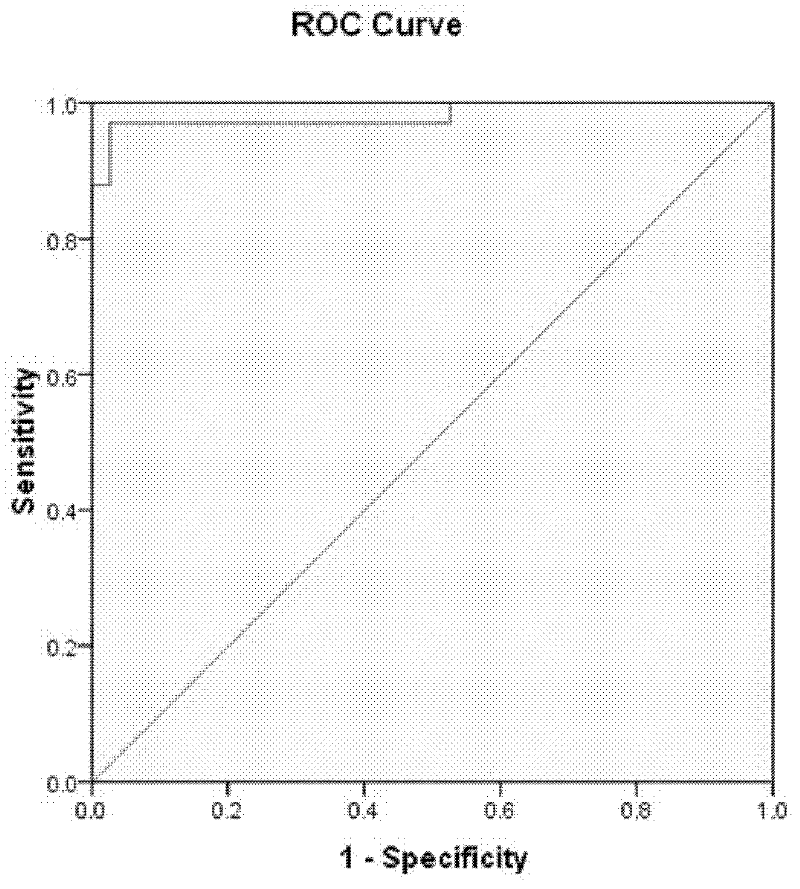
[0136] The 35 markers with significant differences obtained in Example 1 were analyzed, and 14 factors were obtained after logistic regression analysis on the markers with significant differences in expression levels: sTNFR2, Flt3Ligand, Fractalkine, IL1ra, IL2, MDC , MIP1alpha, SDF1alphabeta, TARC, TRAIL, SCF, CCL20, MIP3alpha and XCL1Lymphotactin; statistical analysis shows that the positive rate of early diagnosis system is 98.6%, and the area under the ROC diagnostic curve is 99.8%. figure 2 .
[0137] Wherein, the concrete composition of kit is:
[0138] (1) 96-well filter plate and two sealing membranes; purchased from Millipore Company, item number MX-PLATE;
[0139] (2) Standards of protein antibodies in the kit; purchased from Millipore Company, catalog numbers MXH8060, MXH8062, MXH8063, LHSP-8063 and HSCR-8032;
[0140] (3) Quality control control; purcha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com