Shikonin octyl methoxycinnamate derivant as well as synthesis method and application thereof
A technology of cinnamate esters and derivatives is applied in the field of preparation and application in tumor suppression, and can solve problems such as changing the related properties of shikonin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0037] Example 1: Synthetic method of formula I class shikonin cinnamate derivatives
[0038]Dissolve 50mmol of shikonin and 50mmol of the corresponding cinnamic acid in 20mL of refined dichloromethane, and continue to add a catalytic amount of N,N-dicyclohexylcarbodiimide (DCC) and 4 - Dimethylaminopyridine (DMAP), TLC follow-up detection to generate corresponding ester derivatives. Add an appropriate amount of silica gel to concentrate the solvent under reduced pressure, and perform column chromatography with ethyl acetate:petroleum ether=1:7 to obtain the corresponding shikonin carboxylate derivatives.
[0039] The reaction takes the synthesis of 2,4-dichlorocinnamic acid shikonin as an example:
[0040]
[0041] Compound 1
[0042] Compounds 2-21 can be obtained in the same way.
[0043]
[0044] The physicochemical data of the corresponding compounds are as follows:
[0045] Compound 1: 1 H NMR (500MHz, CDCl 3 )δ: 12.646 (s, 1H, -OH); 12.458 (s, 1H, -OH); 7.76...
example 2
[0064] Example 2. Application of Formula I Class Shikonin Cinnamate Derivatives
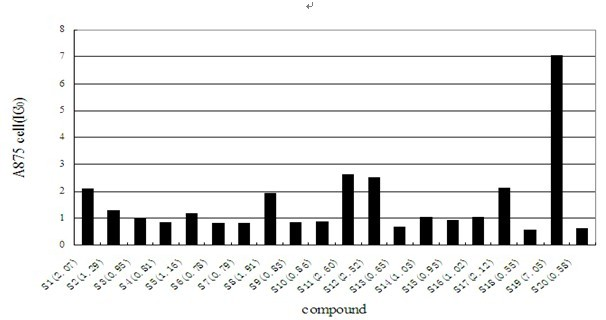
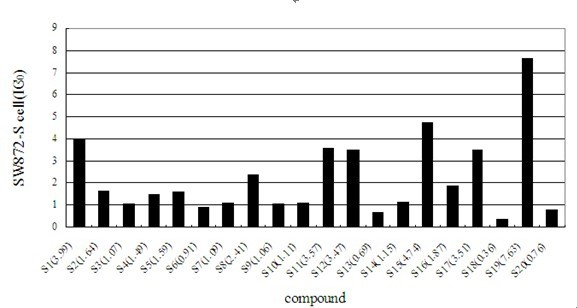
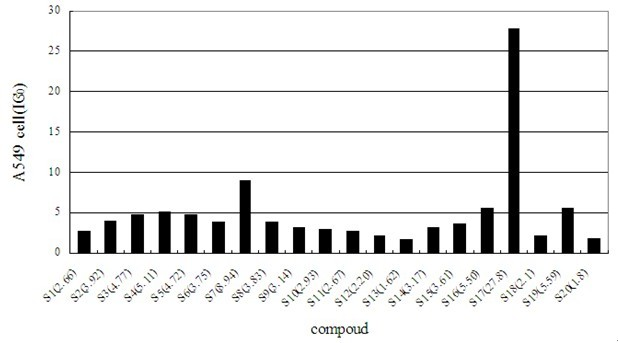
[0065] We studied the anti-tumor activity of Shikonin cinnamic acid derivatives of formula I, selected tumor cells A875, SW872-S and A549 as detection cells, measured their absorbance at 570nm with a microplate reader by MTT method and calculated OD value.
[0066] IC 50 The value calculation method is as shown in the formula:
[0067] Cell inhibition rate (%)=(OD value of control group-OD value of experimental group) / OD value of control group×100%
[0068] The results show that most of the shikonin cinnamate derivatives have very good inhibitory effect on anti-tumor activity. The corresponding results are attached Figure 1-3 .
[0069] The results of the activity of the three cell lines showed that the derivatives of shikonin cinnamate had obvious inhibitory activity on the cancer cell lines A875, SW872-S and A549, and the compound with the best activity was 3,4-difluorocinnamic acid shiko...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com