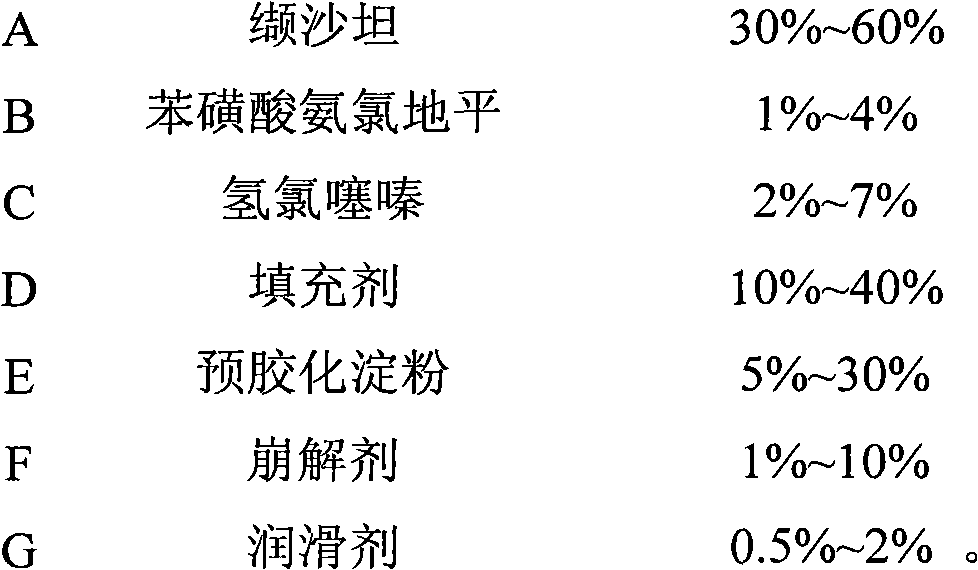
Oral tablet containing valsartan, hydrochlorothiazide and amlodipine besylate
A technology for oral tablet of amlodipine besylate and hydrochlorothiazide, applied in the field of medicine, can solve the problems of easy moisture absorption of tablets, adverse effects on product production, poor product quality and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Wherein 6.9g of amlodipine besylate contains 5g of amlodipine free base.
[0041] Weigh the corresponding materials according to the above prescription, mix A~F in the general blender, then granulate with a dry granulator, add G to mix, use a rotary tablet machine to compress, coat, and then use Aluminum-plastic packaging, to investigate the dissolution rate and stability under accelerated conditions.
Embodiment 2
[0044] Wherein 6.9g of amlodipine besylate contains 5g of amlodipine free base.
[0045] Weigh the corresponding materials according to the above prescription, mix A~G in the general blender, then granulate with a dry granulator, add H to mix, use a rotary tablet machine to compress, and coat. Of which hydrochlorothiazide D 90 =51 μm, valsartan D 90 =215 μm, amlodipine D90=156 μm.
Embodiment 3
100.0%
[0049] Wherein 6.9g of amlodipine besylate contains 5g of amlodipine free base.
[0050] Weigh the corresponding materials according to the above prescription, mix A~F in the general blender, then granulate with a dry granulator, mix G~I, mix with the previous A~F granules, add J After mixing, the tablets are compressed using a rotary tablet press. Of which hydrochlorothiazide D 90 =31 μm, valsartan D 90= 158 μm, amlodipine D90 = 156 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com