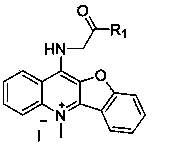
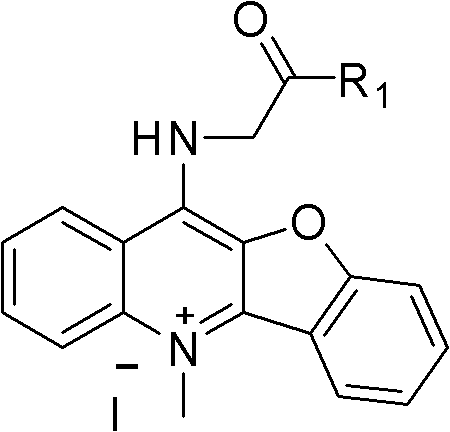
Peptide derivative of benzfuran quinoline and preparation method thereof and application thereof as antitumor medicament
A technology of benzofuran quinoline and its derivatives, which is applied to the peptide derivatives of benzofuran quinoline and its preparation, and is used in the application field of anticancer drugs, which can solve the problems of application restrictions and limited resources. Achieve the effects of high safety, broad market prospects and significant inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
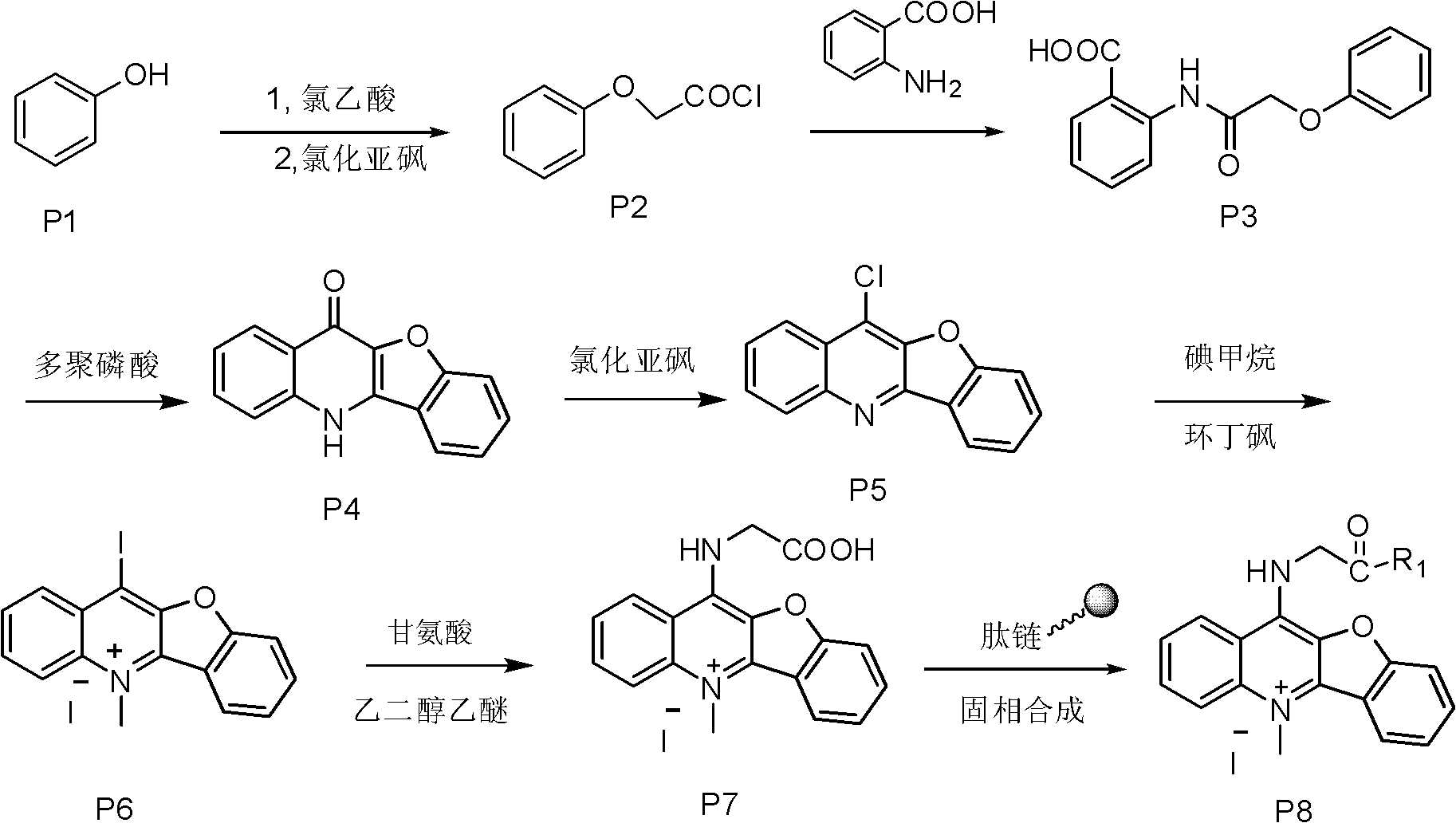
[0026] Example 1: Synthesis of compound P7
[0027] Dissolve 0.3 mol chloroacetic acid in 60 ml water, adjust the pH to 9 with sodium hydroxide, add 0.2 mol phenol, reflux at 100°C, and then add thionyl chloride for chlorination to obtain P2, distill off thionyl chloride The solvent obtains a brown liquid, which is then subjected to condensation reaction with anthranilic acid to obtain P3, and then PPA is preheated to 130° C. and added to P3 for compound reaction to obtain compound P4. P4 and thionyl chloride are refluxed for chlorination reaction at 80° C. to obtain compound P5, and then methyl iodide is performed in a system with sulfolane as a solvent to obtain compound P6. Then 0.05 mol of P6 and 0.25 mol of glycine were refluxed at 120° C. for 4 h with ethylene glycol ether as the solvent, and purified by silica gel chromatography with methanol / dichloromethane as the eluent to finally obtain the light green compound P7.
[0028] Yield: 41%; 1 H NMR (400MHz, DMSO) δ 9.75 (t, J...
Embodiment 2
[0031] Example 2: Synthesis of Compound R
[0032] Dissolve 0.2mol Fmoc-Arg(Pbf)-OH amino acid in DMF (dimethylformamide) solvent, add 0.8mol HOBT(1-hydroxybenzotriazole), 0.8molDIC(N,N-diisopropyl) Carbodiimide) two condensation reagents (condensation reagent ratio is 1:1), react with the deprotected Rink Amide AM resin in a solid phase reactor for 3 hours, and then use 25% piperidine to remove the Fmoc group. The amino acid side chain is obtained. Then it was condensed with P7 in HOBT and DIC in DMF solution, 24h, the resin was removed with trifluoroacetic acid, collected, and purified by preparative high performance chromatography, and finally a light yellow solid R was obtained.
[0033] Yield: 32%; 1 H NMR (400MHz, DMSO) δ 9.71 (t, J = 6.4 Hz, 1H), 8.71 (d, J = 8.5 Hz, 1H), 8.63 (d, J = 8.2 Hz, 1H), 8.56 (d, J = 8.2 Hz, 1H), 8.43 (d, J = 9.0 Hz, 1H), 8.15-8.07 (m, 1H), 7.87 (ddd, J = 21.5, 15.3, 8.0 Hz, 3H), 7.64 (t, J = 7.4Hz, 2H), 7.49(s, 1H), 7.13(s, 2H), 4.79(d, J=6.4Hz...
Embodiment 3
[0036] Example 3: Synthesis of compound GG
[0037] The method is the same as in Example 2, except that Fmoc-Gly-OH is used, and the amino acid is connected to Rink Amide AM resin twice. Finally, it was purified by preparative high performance chromatography to obtain white solid GG.
[0038] Yield: 29%; 1 H NMR (400MHz, DMSO) δ 9.72 (t, J = 6.5 Hz, 1H), 8.70 (t, J = 8.4 Hz, 2H), 8.64 (d, J = 8.2 Hz, 1H), 8.44 (d, J =8.9Hz, 1H), 8.12(dd, J=9.8, 6.4Hz, 2H), 7.93(t, J=5.9Hz, 2H), 7.87-7.77(m, 1H), 7.65(ddd, J=8.2, 5.8, 2.4 Hz, 1H), 7.23 (s, 1H), 7.02 (s, 1H), 4.78 (d, J = 6.4 Hz, 2H), 4.59 (s, 3H), 3.83 (d, J = 5.7 Hz, 2H), 3.60 (d, J=5.8 Hz, 2H); C 22 H 22 N 5 O 4 + , LC-MS m / z: 420[M+H] + .
[0039]
[0040] Compound GG
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com