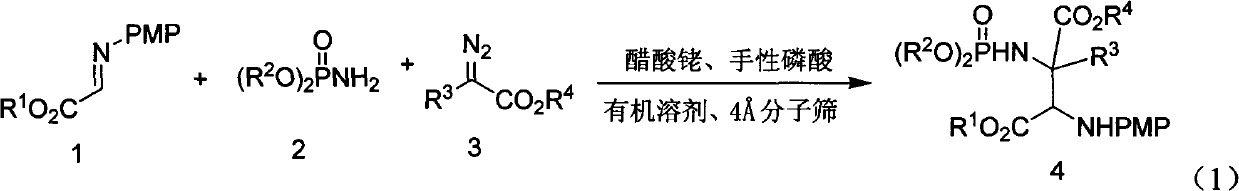
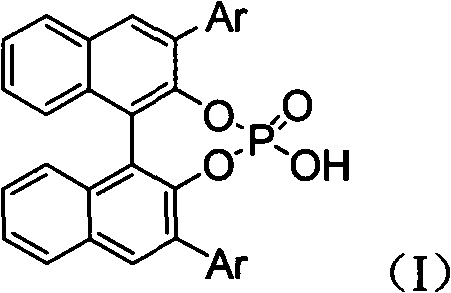
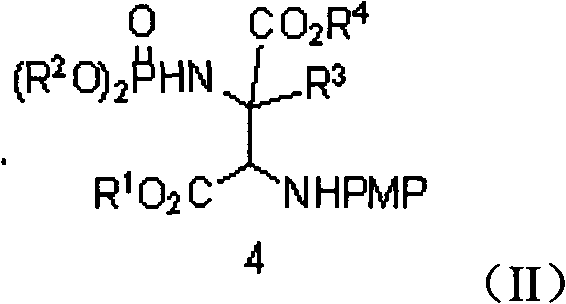
Alpha-quaternary carbon contained alpha, beta-diamino acid derivative, synthetic method thereof and application thereof
A synthesis method and technology of diamine acid, applied in the α field of α-position quaternary carbon, can solve the problems of poor substrate universality, large amount of catalyst, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069]
[0070] Weigh 2-p-methoxyphenylimine ethyl acetate (0.25mmol), rhodium acetate (0.005mmol), chiral phosphoric acid catalyst (Ar=9-phenanthrenyl) (0.005mmol), aminophosphoric acid di(2,6 -xylene) ester (0.3mmol), Molecular sieves (100 mg) were placed in a small test tube reactor, and 6 ml of redestilled toluene was added. Weigh phenyldiazoacetate methyl ester (0.3mmol) and dissolve it in 5ml redistilled toluene, and inject it into the reaction system through a peristaltic pump for 1 hour at room temperature. After the reaction is completed, filter, and the filtrate is rotary evaporated at 40°C to remove the solvent. Then by column chromatography (eluent: petroleum ether: ethyl acetate = 1: 30 ~ 1: 10) to separate the optically active trans α-position quaternary carbon α, β-diamine derivative pure product II -1. Yield 66%, d.r. value 17:1, ee value 95%.
[0071] 1 H NMR (400MHz, CDCl 3 ): 7.38-7.40(m, 2H), 7.20-7.22(m, 3H), 6.89-6.97(m, 6H), 6.75(d, J=9.2Hz, 2H)...
Embodiment 2
[0073]
[0074] Weigh 2-p-methoxyphenylimine ethyl acetate (0.25mmol), rhodium acetate (0.025mmol), the same chiral phosphoric acid catalyst (Ar=9-phenanthrenyl) (0.025mmol) as in Example 1, amino Di(2,6-xylyl)phosphate (0.75mmol), Molecular sieves (100 mg) were placed in a small test tube reactor, and 6 ml of redestilled toluene was added. Weigh methyl p-fluorophenyldiazoacetate (0.75mmol) and dissolve it in 5ml redistilled toluene, and inject it into the reaction system through a peristaltic pump for 1 hour at room temperature. After the reaction is completed, filter and remove the filtrate by rotary evaporation at 40°C Solvent, and then separated by column chromatography (eluent: petroleum ether: ethyl acetate = 1: 30 ~ 1: 10) to obtain optically active trans α-position quaternary carbon α, β-diamine derivative pure Product II-2. Yield 55%, d.r. value 15:1, ee value 91%.
[0075] 1 H NMR (400MHz, CDCl 3 ): 7.32-7.35(m, 2H), 6.96-6.99(m, 3H), 6.90(s, 3H), 6.84(t, J=...
Embodiment 3
[0077]
[0078] Weigh 2-p-methoxyphenylimine ethyl acetate (0.25mmol), rhodium acetate (0.0125mmol), the same chiral phosphoric acid catalyst as in Example 1 (Ar=3,5-ditrifluorophenyl) ( 0.0125mmmol), bis (2,6-xylyl) phosphoramidate (0.5mmol), Molecular sieves (100 mg) were placed in a small test tube reactor, and 6 ml of redestilled toluene was added. Weigh methyl p-chlorophenyldiazoacetate (0.5mmol) and dissolve it in 5ml redistilled toluene, and inject it into the reaction system through a peristaltic pump for 1 hour at room temperature. After the reaction is completed, filter and remove the filtrate by rotary evaporation at 40°C Solvent, and then separated by column chromatography (eluent: petroleum ether: ethyl acetate = 1: 30 ~ 1: 10) to obtain optically active trans α-position quaternary carbon α, β-diamine derivative pure Product II-3. Yield 55%, d.r. value 23:1, ee value 90%.
[0079] 1 H NMR (400MHz, CDCl 3 ): 7.25-7.28(d, J=9.2Hz, 2H), 8.8(d, J=8.8Hz, 2H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com