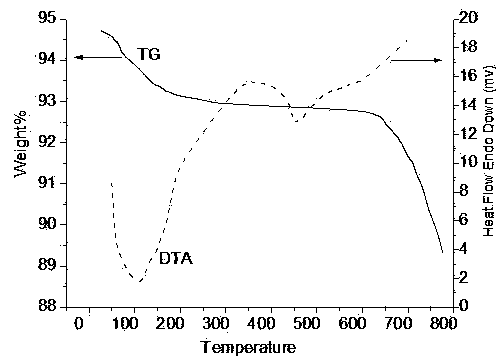
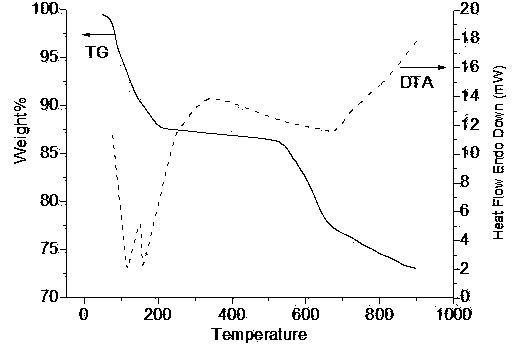
Nanometer vanadium catalyst for preparing sulfuric acid through oxidizing SO2 and preparation method thereof
A sulfur dioxide, oxidation technology, applied in physical/chemical process catalysts, chemical instruments and methods, inorganic chemistry and other directions, can solve the problems of high ignition temperature, difficult reaction control, etc., achieve high low temperature activity, easy control of reaction temperature, The effect of improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Sample composition: V 2 o 5 4%, 0.01% of cerium nitrate, 1% of cesium hydroxide, 0.01% of lanthanum nitrate, 0.5% of surfactant hexadecyltrimethylammonium bromide and 0.7% of organic macromolecular compound polyethylene glycol, and the rest is refined silicon algal earth.
[0043] Using the traditional production process, the diatomite is separated by water, treated with acid, filtered, washed and dried to obtain refined diatomite for use. will V 2 o 5 , KOH are fully dissolved in the same reaction tank, after mixing evenly, add organic polymer compounds and surfactants, and fully react to obtain KVO 3 or NaVO 3 solution; while stirring, the solution and CsOH, lanthanum nitrate, and cerium nitrate are added dropwise to dilute sulfuric acid in a downstream manner, wherein the mass percentage concentration of dilute sulfuric acid is 50%, and the pH value is controlled to be 2 to 4. The neutralization temperature 25°C, at this time, the V produced by the reaction o...
Embodiment 2
[0045] Sample composition: V 2 o 5 5.5%, cerium oxide 5%, cesium sulfate 10%, lanthanum oxide 5%, surfactant sodium dodecylbenzenesulfonate, sodium dodecylsulfonate 0.5% each and organic polymer polypyrrolidone 0.5%, Carboxymethyl cellulose is 0.5%, and the rest is refined diatomaceous earth.
[0046] will V 2 o 5 , NaOH is added to the reaction tank to dissolve organic polymer compounds and surfactants, and fully react to obtain NaVO 3 Solution b; while stirring, drop solution b into dilute sulfuric acid in a downstream manner, control the pH value to 2-4, and control the neutralization temperature to 20°C, and react to form nano-scale homogeneous liquid B, in which the dilute sulfuric acid mass The percentage concentration is 70%; the solution B is quickly and evenly sprayed into the refined diatomite in the form of spray, and fully mixed with sodium sulfate, phosphoric acid, lanthanum oxide, cesium sulfate, cerium oxide, and sulfur into the roller. Grinding for 0.5 h...
Embodiment 3
[0048] Sample composition: V 2 o 5 4.5%, cerium nitrate 2.5%, cesium hydroxide 6%, lanthanum nitrate 2.55%, surfactant triethanolamine dodecyl sulfate 1%, organic polymer polystyrene 2%, polyoxyethylene 2%, the rest is Refined diatomaceous earth.
[0049] will V 2 o 5 , KOH is dissolved in the reaction tank, after mixing evenly, add organic polymer compound and surfactant, fully react to get KVO 3 Solution c; while stirring, add solution c, soluble cesium-containing compounds such as CsOH, lanthanum-containing compounds such as lanthanum nitrate or cerium-containing compounds such as cerium nitrate into dilute sulfuric acid in a downstream manner, and control the pH value of 2~ 4. Control the neutralization temperature to ≤30°C, and react to form nano-scale homogeneous liquid C, in which the mass percentage concentration of dilute sulfuric acid is 20-70%; quickly and evenly spray solution C into refined diatomite in the form of spray, And add sodium sulfate, phosphoric ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com