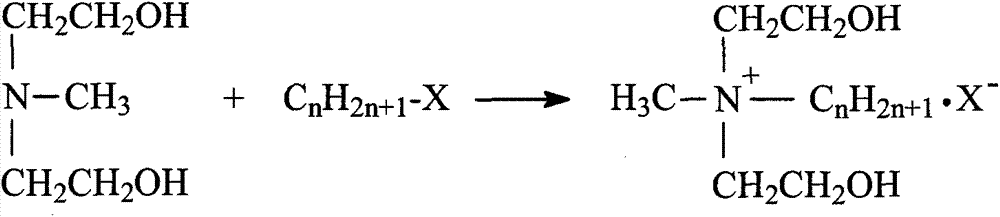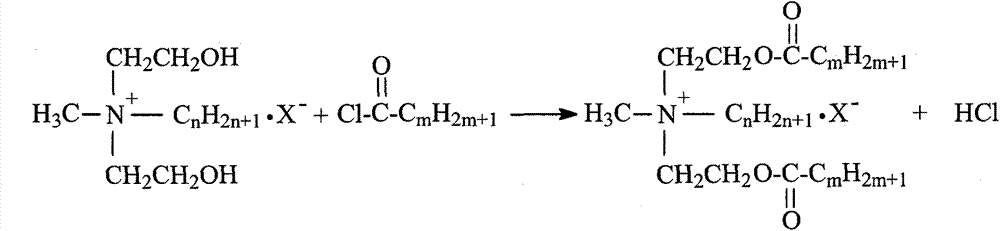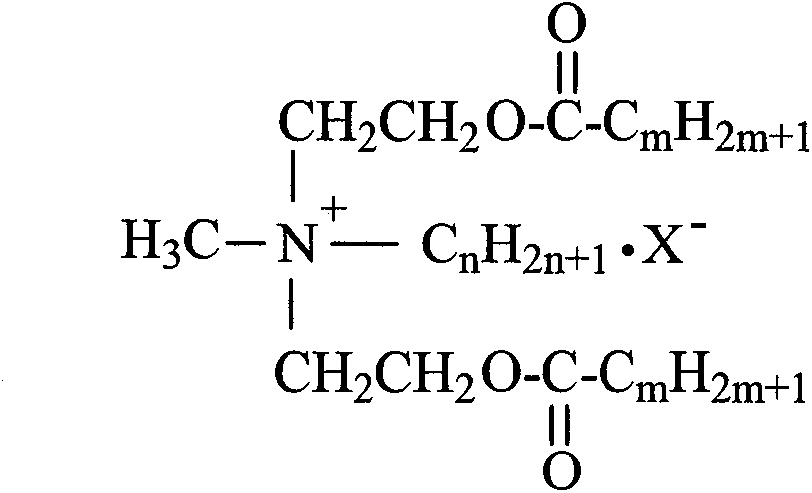Synthetic process of double-long-chain diester quaternary ammonium salt
A diester-based quaternary ammonium salt and a synthetic process technology, applied in the preparation of amino hydroxyl compounds, the preparation of organic compounds, organic chemistry, etc., can solve the problems of high toxicity, low content of double long-chain ester amines, carcinogenicity, etc., and achieve no The effects of three wastes generation, mild reaction conditions, and enhanced degradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Weigh 11.92g (0.1mol) of N-methyldiethanolamine and 14.17g (0.13mol) of bromoethane into a 250mL three-necked flask, add 30mL of methanol as a solvent, and heat it in a water bath to 35°C for constant temperature reaction Stop at 4h, remove methanol by rotary evaporation, use ethyl acetate-methanol mixed solvent to recrystallize to obtain white crystal substance N-methylethyldihydroxyethylammonium bromide; weigh N-methylethyldihydroxyethylammonium bromide Ammonium 11.41g (0.05mol) was added to a 250mL three-necked flask, an appropriate amount of solvent chloroform and acid agent triethylamine were added, and 30.30g (0.10mol) of stearyl chloride was slowly added dropwise in an ice-water bath, and the temperature rose naturally after the addition Reaction at room temperature for 8h, stop the reaction. The solvent was removed by rotary evaporation, and the white solid product N-methyl ethyl distearate ethyl ammonium bromide was obtained by recrystallization thre...
Embodiment 2
[0022] Example 2: Weigh 11.92g (0.1mol) of N-methyldiethanolamine and 14.17g (0.13mol) of bromoethane, add them to a 250mL three-necked flask, add 35mL of ethanol as a solvent, and heat in a water bath to 45°C for constant temperature reaction Stop at 5h, remove ethanol by rotary evaporation, use ethyl acetate-methanol mixed solvent to recrystallize to obtain white crystal substance N-methylethyldihydroxyethylammonium bromide; weigh N-methylethyldihydroxyethylammonium bromide Ammonium 11.4lg (0.05mol) was added to a 250mL three-necked flask, an appropriate amount of solvent chloroform and acid agent triethylamine were added, and 33.33g (0.11mol) of stearyl chloride was slowly added dropwise in an ice-water bath, and the temperature rose naturally after the addition To room temperature reaction 10h, stop the reaction. The solvent was removed by rotary evaporation, and the white solid product N-methyl ethyl distearate ethyl ammonium bromide was obtained by recrystallization thre...
Embodiment 3
[0023] Example 3: Weigh 11.92g (0.1mol) of N-methyldiethanolamine and 15.26g (0.14mol) of bromoethane into a 250mL three-necked flask, add 40mL of acetone as a solvent, and heat it in a water bath to 50°C for constant temperature reaction Stop at 4h, remove acetone by rotary evaporation, and use ethyl acetate-methanol mixed solvent to recrystallize to obtain white crystal substance N-methylethyl dihydroxyethyl ammonium bromide; weigh N-methyl ethyl dihydroxyethyl ammonium bromide Ammonium 11.4lg (0.05mol) was added to a 250mL three-necked flask, an appropriate amount of solvent chloroform and acid agent triethylamine were added, and 30.30g (0.10mol) of stearyl chloride was slowly added dropwise in an ice-water bath, and the temperature rose naturally after the addition To room temperature reaction 10h, stop the reaction. The solvent was removed by rotary evaporation, and the white solid product N-methyl ethyl distearate ethyl ammonium bromide was obtained by recrystallization ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com