Synthesis method of anacardol and butyl ether composition
A technology of cardanol butyl ether and a synthesis method, applied in the field of ether synthesis, can solve problems such as deepening of color and luster, and achieve the effect of eliminating allergens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
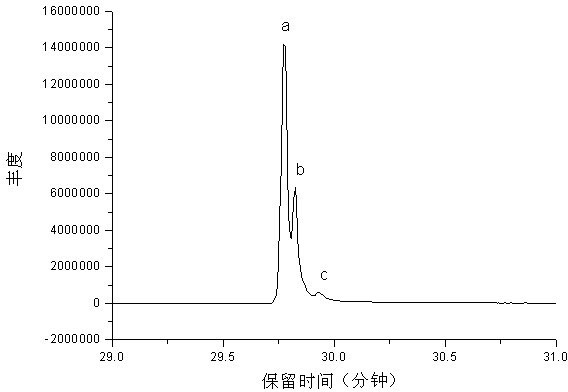
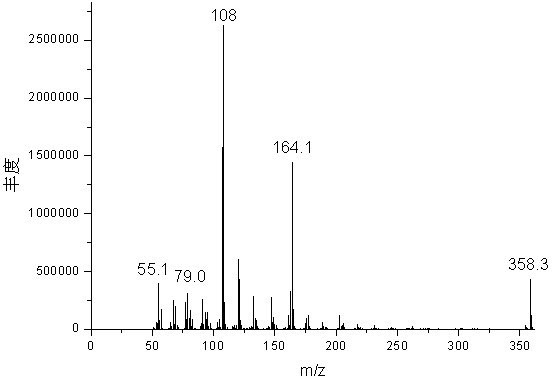
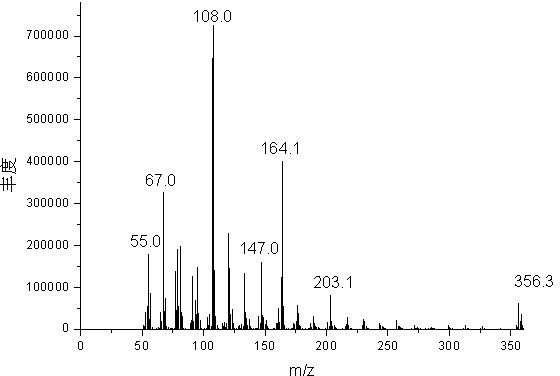
[0027] Mix 1 mole of fresh cardanol (Shandong Tengzhou Yalante Biomaterial Co., Ltd.), 1 mole of chlorobutane (Yixing Changjili Chemical Co., Ltd.) and 0.5 mole of potassium carbonate (Chengdu Kelong Chemical Reagent Instrument Factory) ) into a 1000 ml four-neck flask equipped with a nitrogen inlet, a thermometer, a stirrer and a reflux condensing device for mixing and stirring, and the nitrogen outlet was sealed with a funnel upside down in water. The flask was purged of air by bubbling nitrogen gas for 10 minutes. The temperature was raised to 80°C and maintained for 6 hours. Heating in a water bath at 96°C, using a rotary evaporator and a circulating water vacuum pump to distill off the water generated by the reaction under reduced pressure, and using a Buchner funnel to filter out the salt to obtain 340 g of light yellow and transparent cardanol butyl ether, with a yield of 95.5%.
Embodiment 2
[0029] Mix 1 mole of fresh cardanol (Shandong Tengzhou Yalante Biomaterials Co., Ltd.), 1.5 moles of n-chlorobutane (Yixing Changjili Chemical Co., Ltd.) and 1 mole of potassium carbonate (Chengdu Kelong Chemical Reagent Instrument Factory) ) into a 1000 ml four-neck flask equipped with a nitrogen inlet, a thermometer, a stirrer and a reflux condensing device for mixing and stirring, and the nitrogen outlet was sealed with a funnel upside down in water. The flask was purged of air by bubbling nitrogen gas for 10 minutes. The temperature was raised to 120°C and held there for 2 hours. Heated in a water bath at 96°C with a rotary evaporator and a circulating water vacuum pump to distill off excess n-butyl chloride and the water generated by the reaction, and remove the salt by suction filtration with a Buchner funnel to obtain 350 g of light yellow transparent cardanol butyl ether. Yield 98.3%.
Embodiment 3
[0031] Mix 1 mole of fresh cardanol (Shandong Tengzhou Yalante Biomaterials Co., Ltd.), 1.5 moles of n-chlorobutane (Yixing Changjili Chemical Co., Ltd.), 200 ml of dimethylformamide and 1 mole of sodium carbonate ( Tianjin Bodi Chemical Factory) was added into a 1000 ml four-neck flask equipped with a nitrogen inlet, a thermometer, a stirrer and a reflux condensing device for mixing and stirring, and the nitrogen outlet was sealed with a funnel upside down in water. The flask was purged of air by bubbling nitrogen gas for 10 minutes. The temperature was raised to 120°C and held there for 2 hours. Heated in a water bath at 96° C. with a rotary evaporator and a circulating water vacuum pump to distill off excess n-butyl chloride and the water generated by the reaction, and remove the salt by suction filtration with a Buchner funnel to obtain 347 g of light yellow transparent cardanol butyl ether. Yield 97.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com