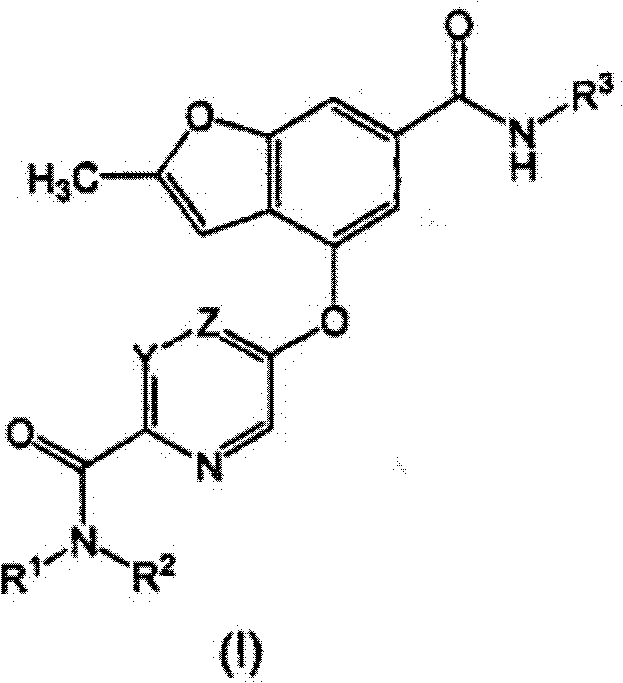
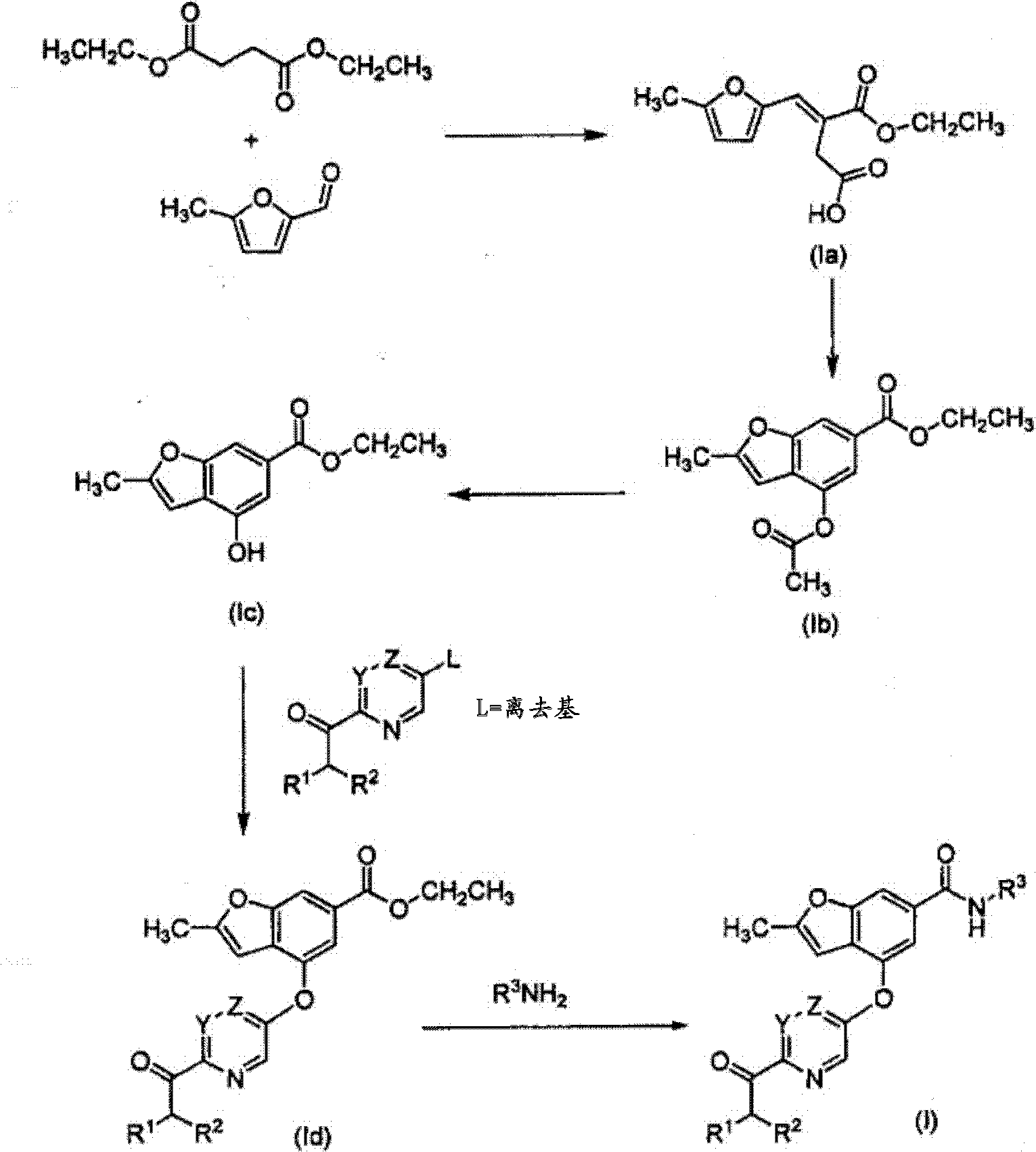
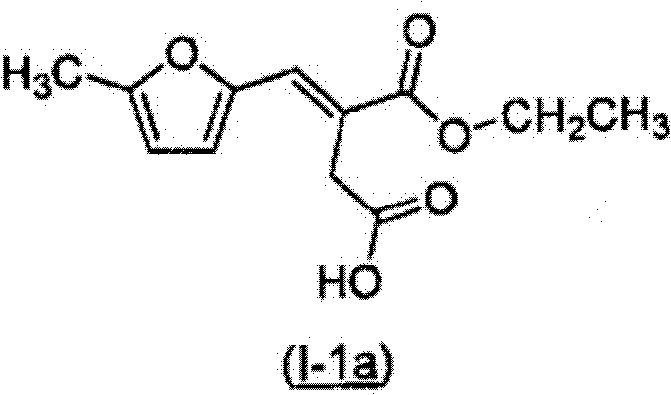
Benzofuranyl derivatives used as glucokinase inhibitors
A technology of benzofuran and compounds, applied in the field of benzofuran-based derivatives, can solve problems such as weight gain, induced edema and anemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0069] Unless otherwise specified, raw materials are usually obtained from the following commercial sources, for example: Aldrich Chemicals Co. (Milwaukee, WI), Lancaster Synthesis, Inc. (Windham, NH), Acros Organics (Fairlawn, NJ), Maybridge Chemical Company, Ltd. (Cornwall, UK), Tyger Scientific (Princeton, NJ) and AstraZeneca Pharmaceuticals (UK, London). The following materials can be purchased from corresponding sources:
[0070] 5-Methyl-2-furaldehyde-Sigma-Aldrich (Milwaukee, WI);
[0071] 5-Methyl-2-aminopyrazine-Princeton Bimolecular Research, Inc (Monmouth Junction, NJ);
[0072] 5-Methoxypyrazine-2-amine-Anichem (Monmouth Junction, NJ);
[0073] 5-Chloropyrazine-2-carboxylic acid-Ark Pharma, Inc (Libertyville, IL);
[0074] 1-Methyl-1H-pyrazol-3-ylamine-Matrix Scientific (Columbia, SC);
[0075] 5-Bromo-pyrimidine-2-carboxylic acid-Ark Pharma, Inc (Libertyville, IL).
[0076] General experimental procedure
[0077] At room temperature and 400MHz, in Varian Unity TM NMR spect...
Embodiment 1
[0127] N,N-Dimethyl-5-(2-methyl-6-((5-methylpyrazin-2-yl)carbamoyl-benzofuran-4-yloxy)pyrazine-2- Preparation of formamide (1):
[0128]
[0129] 5-Methyl-2-aminopyrazine (6.8 g, 63 mmol) was dissolved in 70 mL of dimethyl ether and cooled to 0°C. Dimethyl aluminum chloride (131 mmol, 1 M hexane) was added dropwise. The resulting mixture was warmed to room temperature and stirred for 30 minutes. Next, ethyl 4-(5-(dimethylcarbamoyl)pyrazin-2-yloxy)-2-methylbenzofuran-6-carboxylate (I-1d:10.1g , 27.3 mmol) of dimethyl ether solution (70 mL) was added to the active amine solution. The combined solution was heated to reflux overnight. The reaction was cooled on ice and slowly quenched by the dropwise addition of aqueous Rochelle salt (concentrated, 100 mL). The mixture was stirred for 20 minutes. The mixture is separated. The organic layer was washed with aqueous Rochelle salt (30 mL), 1N HCl (30 mL), brine (30 mL), dried over sodium sulfate, and concentrated in vacuo. The cr...
Embodiment 2
[0133] N,N-Dimethyl-5-(2-methyl-6-((5-methylpyrazin-2-yl)carbamoyl)-benzofuran-4-yloxy)pyrimidine-2- Preparation of formamide (2):
[0134]
[0135] At 0℃, Me 2 AlCl (1M hexane solution) (715 mL) was added to a 5-methyl-2-aminopyrazine (38.9 g, 356 mmol) in dimethyl ether solution in a three-necked flask equipped with an overhead stirrer and condenser ( 315mL). The mixture was warmed to room temperature and stirred for 1.5 hours. In a separate flask, ethyl 4-(2-(dimethylcarbamoyl)pyrimidin-5-yloxy)-2-methylbenzofuran-6-carboxylate (I-2a: 52.6g, 142.5 mmol) was dissolved in dimethyl ether (210 mL). This mixture is then added to the complex amine. The gum precipitates and dissipates into a solid when scraping the flask. The resulting reaction was refluxed for 3.5 hours, and HPLC showed that the reaction was 93% complete. 5 liters of Rochelle salt and 2 liters of 2-methyltetrahydrofuran prepared in water were added to the mixture. The reaction mixture was then poured into the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com