Method for separating magnesium and lithium and enriching lithium from salt lake brine
A technology of salt lake brine and brine magnesium, which is applied in the direction of improving process efficiency, can solve the problems of serious equipment corrosion, unfavorable large-scale production, and low product purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
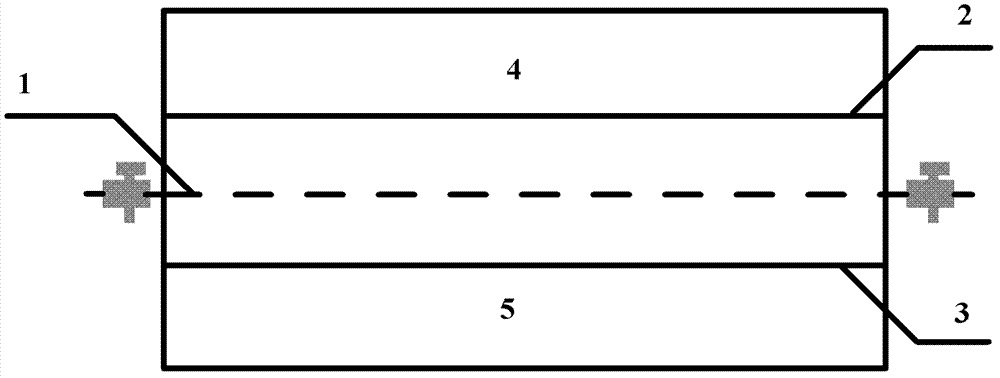
[0045] According to the weight ratio of 20:1:1, 10gFePO 4 Mix ion sieve, 0.5g high-purity graphite and 0.5g PVDF evenly, add N-methylpyrrolidone (NMP) organic solvent to the mixed powder, grind and adjust the slurry, coat the slurry on a graphite plate, and heat it at 110°C Insulated and dried in a vacuum box for 12 hours, the ferric phosphate ion sieve composite membrane was obtained after cooling; the ferric phosphate composite membrane was placed in the brine chamber of the electrodialysis device, and the top view schematic diagram of the electrodialysis device is as follows figure 1 Shown; Add 2L of a salt lake brine into the brine chamber, the main components and contents of the salt lake brine are shown in the following table:
[0046]
[0047] Add 500mL of NaCl solution with a concentration of 20g / L into the lithium salt chamber of the electrodialysis device; use the iron phosphate ion sieve membrane as the cathode, and use the inert graphite in the lithium salt cham...
Embodiment 2
[0050] According to the weight ratio of 90:5:5, 9gFe 0.99 mn 0.01 PO 4 , 0.5g of high-purity graphite and 0.5g of PVDF are mixed evenly, and the mixed powder is added to N-methylpyrrolidone (NMP) organic solvent for grinding and slurrying, and the slurry is sprayed or brushed on the ruthenium-titanium mesh, and the mixture is vacuum-conditioned Heat preservation and drying at 110°C for 10 hours, and obtain an iron phosphate ion sieve composite membrane after cooling.
[0051] Put the iron phosphate composite film in the brine chamber, add 2L salt lake brine, the composition and content of the brine are shown in the following table:
[0052]
[0053] Add 200mL of NaCl solution with a concentration of 50g / L into the lithium salt chamber of the electrodialysis device; use the iron phosphate ion sieve membrane as the cathode, and use the Pt electrode in the lithium salt chamber as the anode, and apply a voltage of 1.0V across the electrodes , after maintaining at 50°C for 10...
Embodiment 3
[0056] By the method of embodiment 2, 3g Fe 0.98 co 0.02 PO 4 Make iron phosphate composite film, put iron phosphate composite film in brine chamber, add 500mL salt lake brine, the composition and content of salt lake brine are shown in the following table:
[0057]
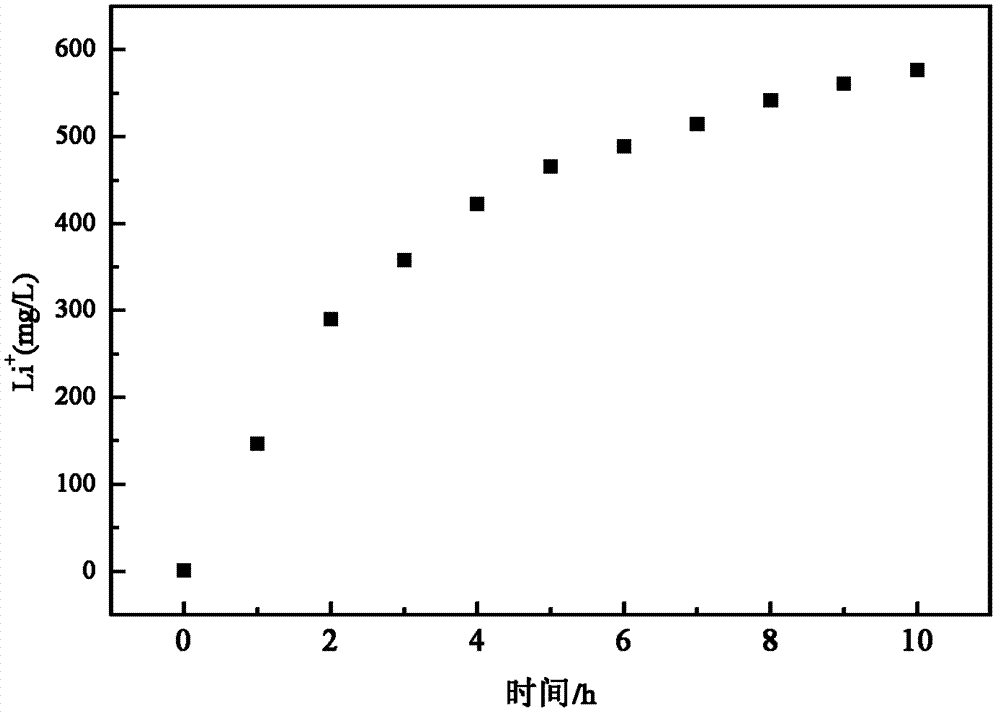
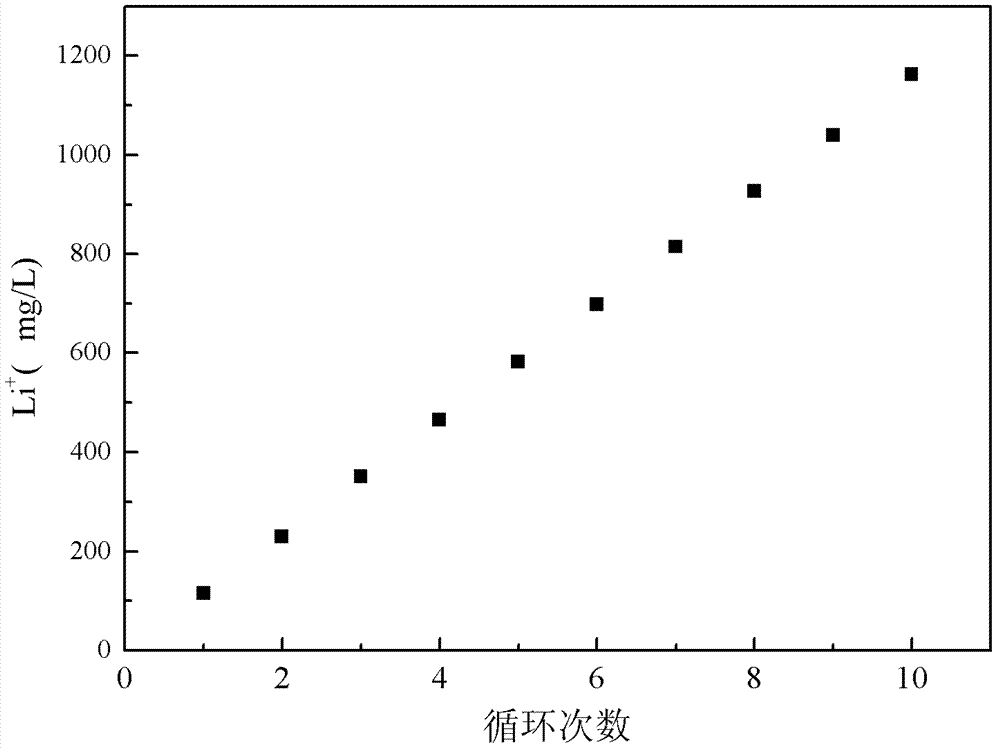
[0058]Add 500mL of NaCl solution with a concentration of 50g / L into the lithium salt chamber, use the iron phosphate composite film as the cathode, and inert graphite as the anode, apply a voltage of 2.0V, and after maintaining it at 80°C for 10h, Li in the brine chamber + The concentration was reduced to 268.4mg / L, Mg 2+ The concentration is 17991mg / L, Fe 0.98 co 0.02 PO 4 ion sieve for Li + The adsorption capacity is 38.6mg / g, for Mg 2+ The adsorption capacity is 1.5mg / g.
[0059] According to the same method in this example, 3g Fe 0.98 co 0.02 PO 4 The ion sieve is made into an iron phosphate composite membrane without lithium intercalation. After the initial lithium intercalation is completed, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com