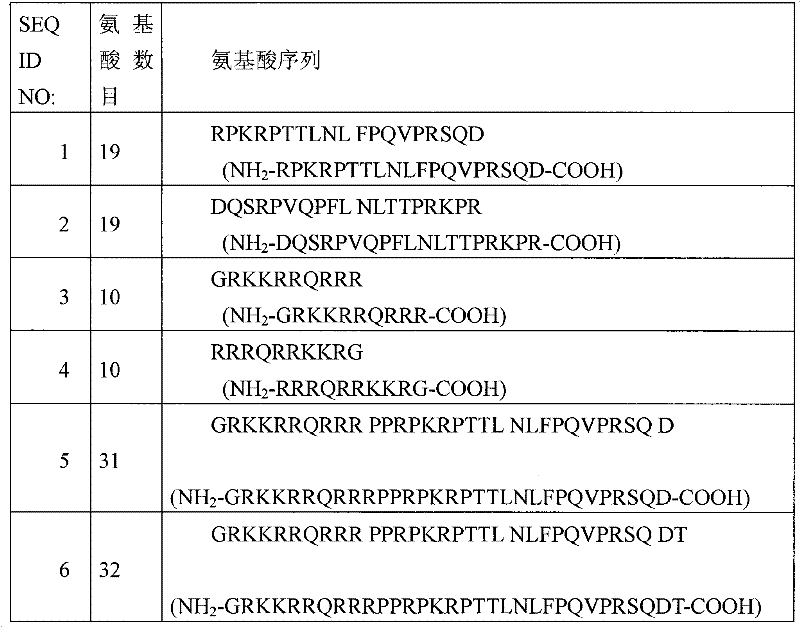
Prophylactic or therapeutic agent for retinal diseases and method for preventing or treating retinal diseases, each comprising jnk (c-jun n-terminal kinase)-inhibiting peptide, and use of the peptide
A technology for retinal diseases and preventive agents, applied in the field of preventing or treating retinal diseases, and preparing preventive or therapeutic agents for retinal diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0137] (Preparation Example 1: Injection)
[0138] In 10mL:
[0139] A peptide 10mg
[0141] Appropriate amount of polysorbate 80
[0142] Appropriate amount of sterile purified water
[0143] Peptide A and other components listed above are dissolved in sterile purified water to prepare injections. Injections containing 0.1 mg, 1 mg or 50 mg of A peptide in 10 ml can be prepared by changing the amount of A peptide added.
preparation Embodiment 2
[0144] (Formulation Example 2: Eye Drops (0.01% (w / v)))
[0145] In 100ml:
[0146]
[0147]
[0148] Peptide A and other ingredients listed above are added to sterile purified water and mixed well to prepare an ophthalmic solution. Drops containing peptide A at concentrations of 0.05% (w / v), 0.1% (w / v), 0.5% (w / v), or 1% (w / v) can be prepared by varying the amount of peptide A added eye drops.
preparation Embodiment 3
[0149] (Formulation Example 3: tablet)
[0150] In 100mg:
[0151]
[0152] Peptide A and lactose are mixed in a mixer. Carboxymethylcellulose calcium and hydroxypropylcellulose were added to the mixture, and the mixture was granulated. The resulting granules were dried and sized, magnesium stearate was added to the sized sized granules and blended, after which the mixture was compressed into tablets in a tablet machine. By changing the amount of peptide A added, tablets containing 0.1 mg, 10 mg or 50 mg of peptide A in 100 mg can be prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com