Method for preparing carbon-hybridized nickel lithium ferrite nano-catalyst
A carbon hybrid ferric acid and nano-catalyst technology, applied in the direction of catalyst activation/preparation, physical/chemical process catalysts, chemical instruments and methods, etc., to achieve the effect of simple and easy operation, large-scale industrial production, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A preparation method of carbon hybrid nickel lithium ferrite nano catalyst, it comprises the following steps:
[0024] 1) Dissolve lithium nitrate, nickel nitrate, iron nitrate and citric acid in 50mL water in a 100mL beaker at room temperature (10-30°C) and stir to prepare lithium nitrate, nickel nitrate, iron nitrate and lemon Clear solutions with acid concentrations of 2mol / L, 1mol / L, 4mol / L, and 7mol / L;
[0025] 2) Place the beaker (with a clear solution inside) in a blast drying oven at 200°C for 8 hours to obtain a foamy intermediate;
[0026] 3) Calcining the intermediate in a muffle furnace at 550°C for 3 hours to obtain lithium nickel ferrite (granular);
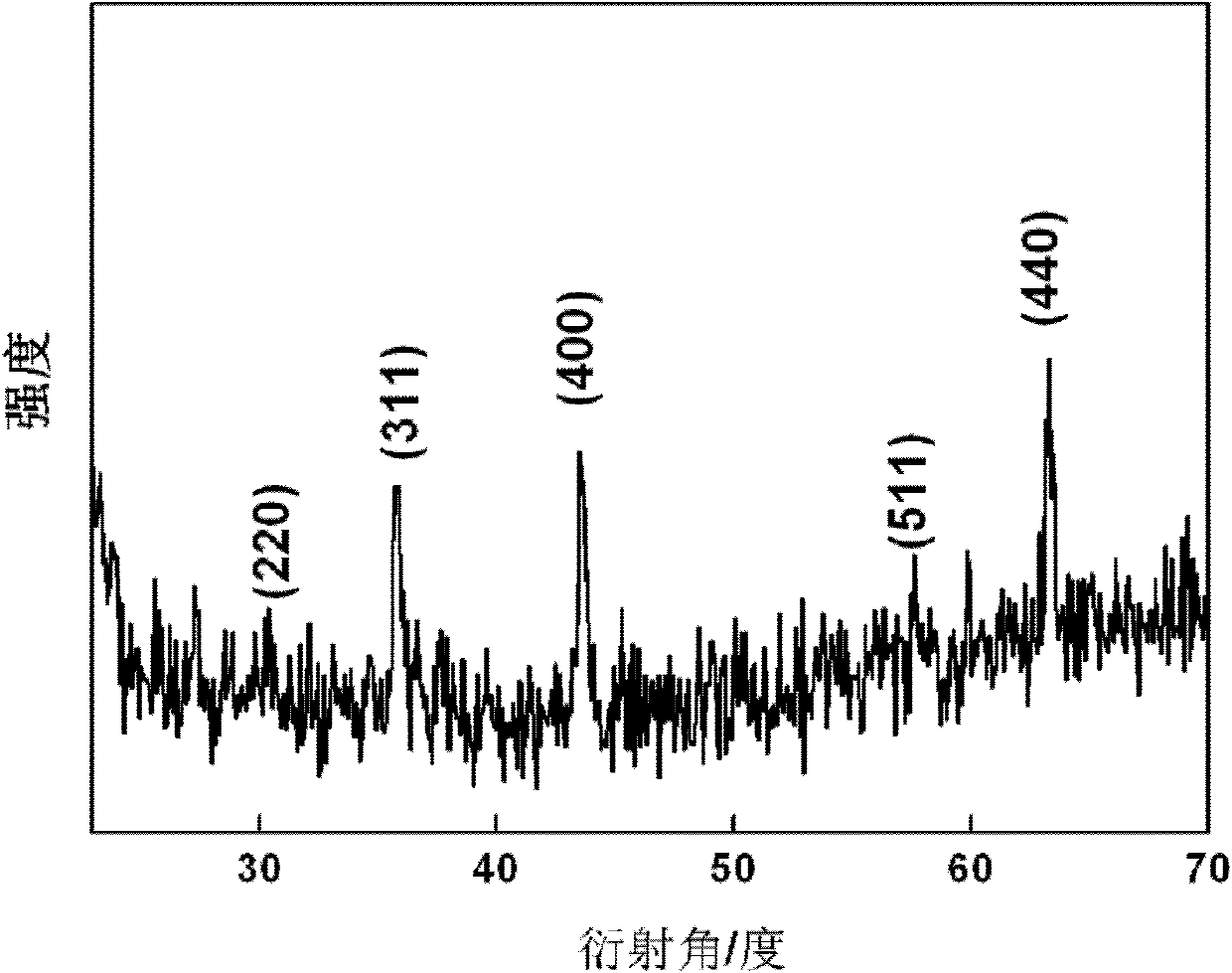
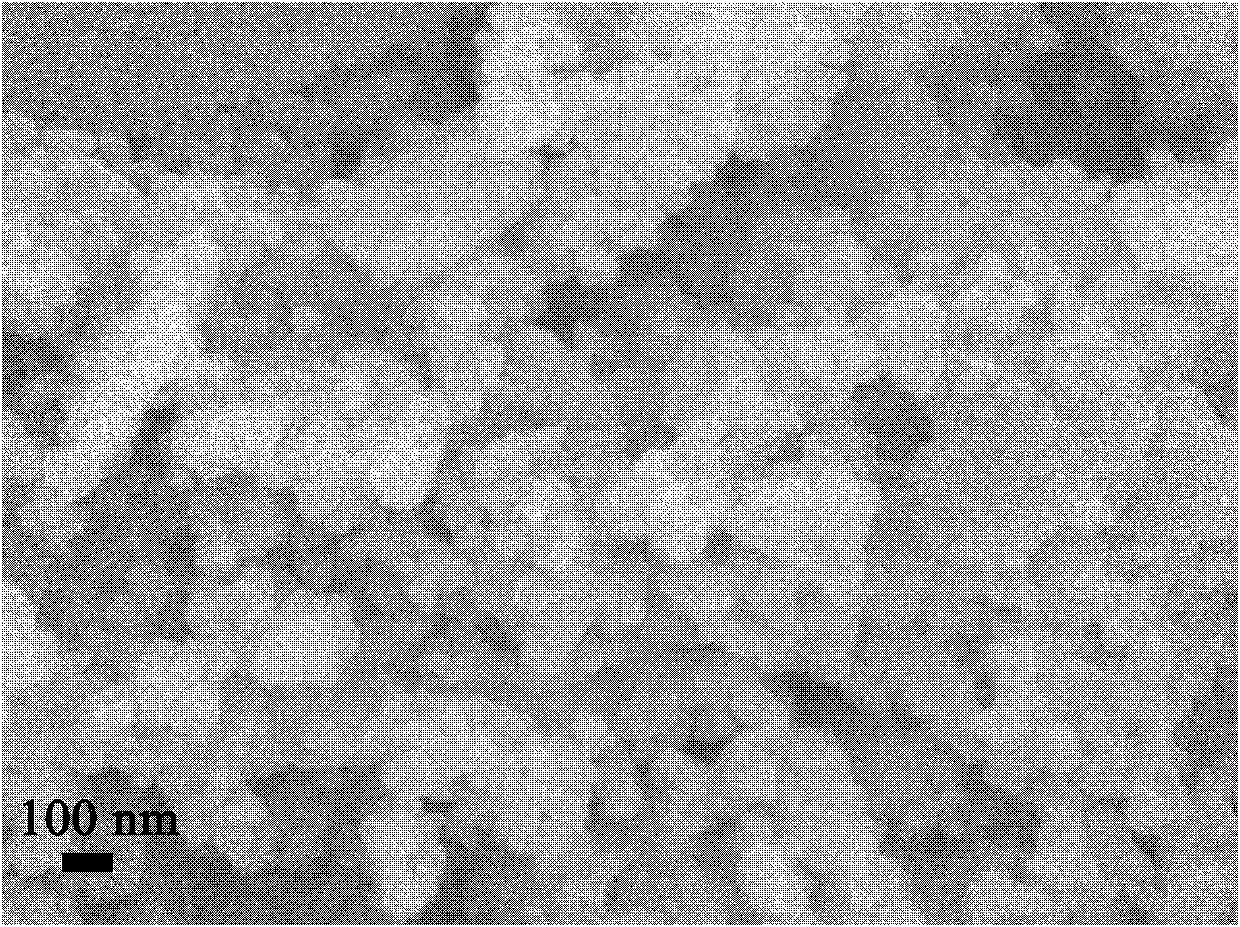
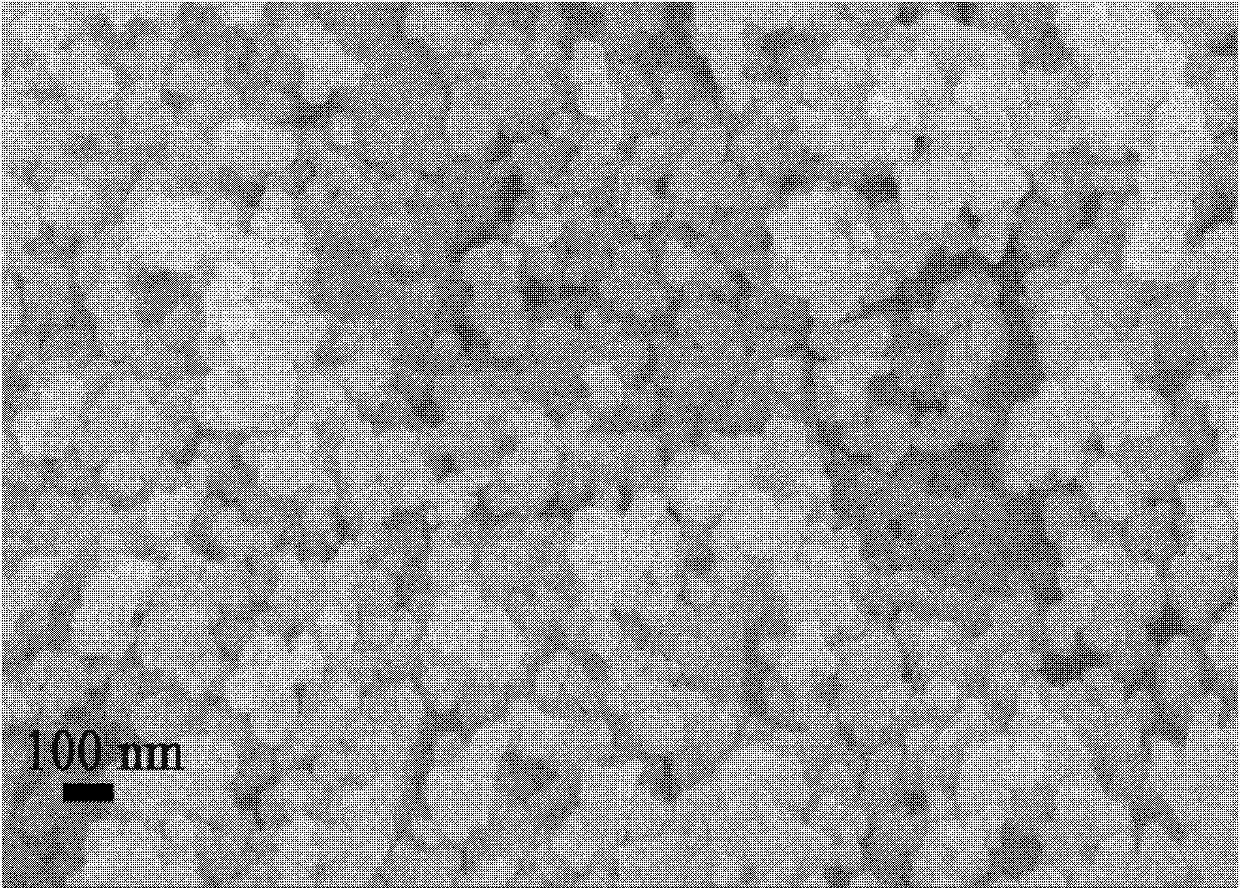
[0027] The XRD diffraction pattern of the obtained lithium nickel ferrite is shown in figure 1 , indicating that the obtained sample is pure nickel-lithium ferrite. Gained nickel-lithium ferrite scanning electron microscope picture is shown in figure 2 .
[0028] 4) After mixing the obtained nickel-lith...
Embodiment 2
[0032] A preparation method of carbon hybrid nickel lithium ferrite nano catalyst, it comprises the following steps:
[0033] 1) Dissolve lithium nitrate, nickel nitrate, iron nitrate and citric acid in 50mL water in a 100mL beaker at room temperature (10-30°C) and stir to make lithium nitrate, nickel nitrate, iron nitrate and lemon nitrate Clear solutions with acid concentrations of 2mol / L, 1mol / L, 4mol / L, and 7mol / L;
[0034] 2) Place the beaker (with a clear solution inside) in a blast drying oven at 160°C for 8 hours to obtain a foamy intermediate;
[0035] 3) Calcining the intermediate in a muffle furnace at 550° C. for 3 hours to obtain lithium nickel ferrite.
[0036] 4) After mixing the obtained nickel-lithium ferrite sample with glucose at a mass ratio of 2:1, add it to a polytetrafluoroethylene reactor (i.e., a high-pressure reactor), and conduct a hydrothermal reaction at 160°C for 12 hours. Washing 3 times, drying at 50° C. (drying for 8 hours) to obtain carbon h...
Embodiment 3
[0038] A preparation method of carbon hybrid nickel lithium ferrite nano catalyst, it comprises the following steps:
[0039] 1) At room temperature, in a 100mL beaker, weigh lithium nitrate, nickel nitrate, iron nitrate and citric acid and dissolve them in 50mL of water, stir and dissolve, and prepare lithium nitrate, nickel nitrate, iron nitrate and citric acid. 4mol / L, 2mol / L, 8mol / L, 14mol / L clear solution;
[0040] 2) Place the beaker in a blast oven at 200°C for 8 hours to obtain a foamy intermediate;
[0041] 3) Calcining the intermediate in a muffle furnace at 550° C. for 3 hours to obtain a lithium nickel ferrite sample.
[0042] 4) After mixing the obtained nickel-lithium ferrite sample with glucose at a mass ratio of 1:1, add it to a polytetrafluoroethylene reactor, conduct a hydrothermal reaction at 200°C for 12 hours, wash it with water and ethanol five times after taking it out, and wash it at 50°C Dry (dry for 8 hours) to obtain carbon-coated lithium nickel fe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com