Multifunctional polyurethane medicament carrier as well as preparation method and application thereof
A polyurethane, multifunctional technology, applied in the field of new multifunctional polyurethane drug carrier and its preparation and application, can solve the problems of single function, lack of targeting ability, lack of responsiveness, etc. The effects of various control methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
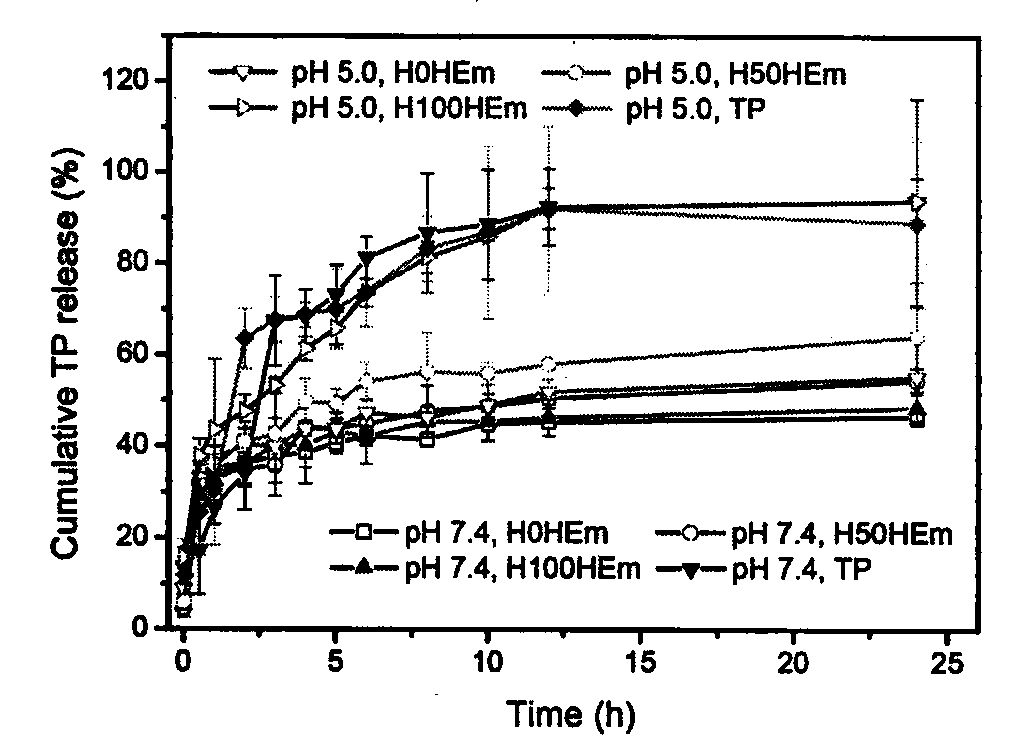
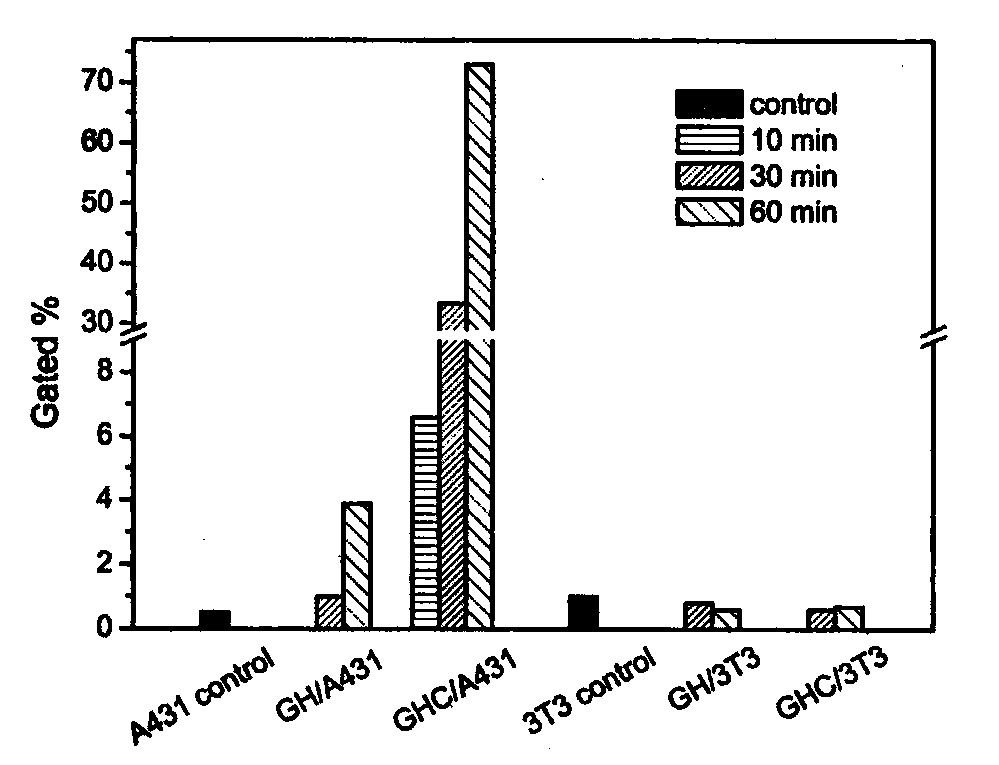
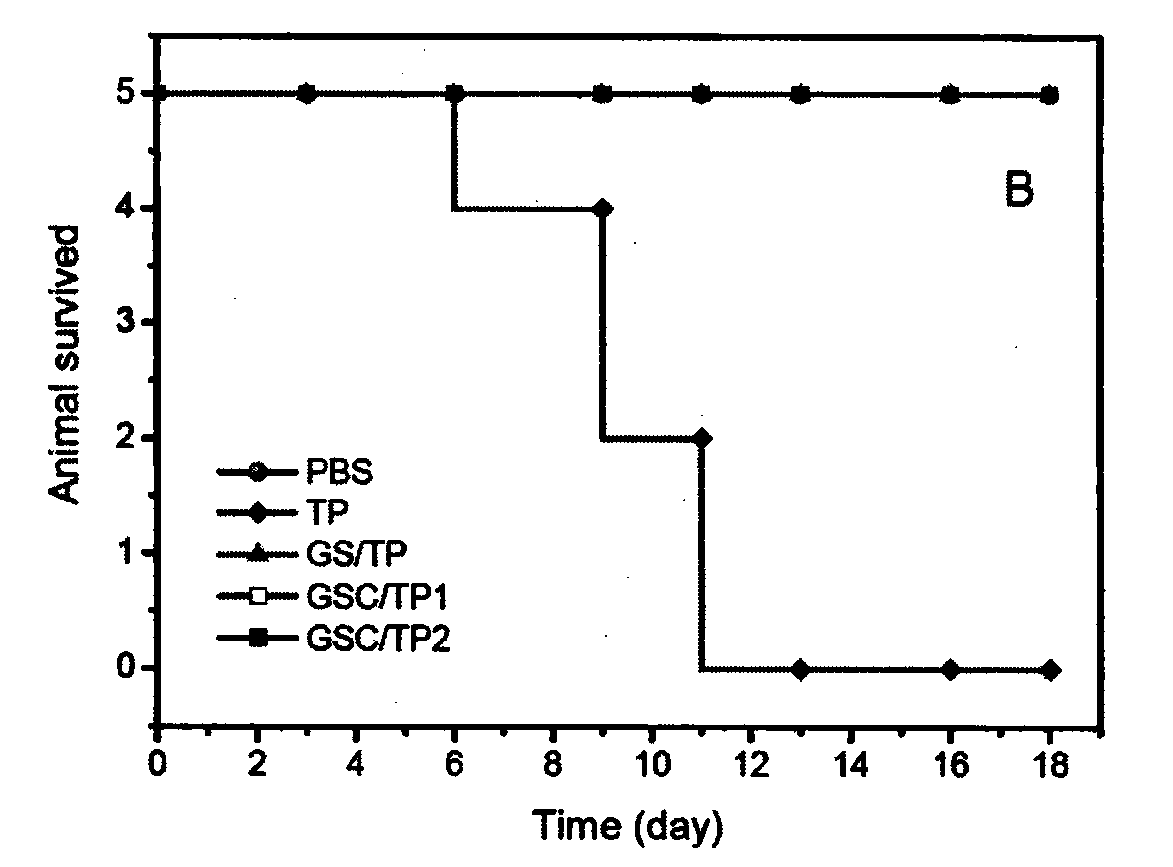
[0065] Add the polymer diol (HPCL) and the comparison polymer diol (PCL) containing the stimuli-sensitive group and the hydrophobic segment into the reaction kettle equipped with a stirrer, a thermometer and a nitrogen protection device, stir and heat up to 60 ℃, vacuum drying for 120 minutes; then lower the temperature to 50 ℃, add aliphatic diisocyanate and solvent, raise the temperature to 60 ℃, prepolymerize for 120 minutes under stirring and nitrogen protection; then add 1‰ stannous octoate catalyst, comparative chain extension Agent (PDO), hydrophilic group-containing chain extender (GA12) and active group-containing chain extender (LGG), reacted for 150 minutes at 25-70°C; finally added the comparative end-capping agent (mPEG), and reacted After 120 minutes, the temperature was raised to 65° C., and the reaction was continued for 300 minutes. After the reaction solution is cooled, stop stirring and nitrogen protection, pour the reaction solution into an excess of ether ...
Embodiment 2
[0067]Add the polymer diol (HPCL) and the comparison polymer diol (PCL) containing the stimuli-sensitive group and the hydrophobic segment into the reaction kettle equipped with a stirrer, a thermometer and a nitrogen protection device, stir and heat up to 60 ℃, vacuum drying for 120 minutes; then lower the temperature to 50 ℃, add aliphatic diisocyanate and solvent, raise the temperature to 60 ℃, prepolymerize for 120 minutes under stirring and nitrogen protection; then add 1‰ stannous octoate catalyst, comparative chain extension Agent (PDO), hydrophilic group-containing chain extender (GA12) and active group-containing chain extender (LGG), reacted for 150 minutes at 25-70°C; finally added the contrast end-capping agent (mPEG) and containing Stimulate the capping agent (HPEG) of the sensitive group, react for 120 minutes, then raise the temperature to 65°C, and continue the reaction for 300 minutes. After the reaction solution is cooled, stop stirring and nitrogen protectio...
Embodiment 3
[0069] Add the polymer diol (HPCL) and the comparison polymer diol (PCL) containing the stimuli-sensitive group and the hydrophobic segment into the reaction kettle equipped with a stirrer, a thermometer and a nitrogen protection device, stir and heat up to 60 ℃, vacuum drying for 120 minutes; then lower the temperature to 50 ℃, add aliphatic diisocyanate and solvent, raise the temperature to 60 ℃, prepolymerize for 120 minutes under stirring and nitrogen protection; then add 1‰ stannous octoate catalyst, comparative chain extension Agent (PDO), hydrophilic group-containing chain extender (GA12) and active group-containing chain extender (LGG), react at 25-70°C for 150 minutes; finally add a capping agent containing a sensitive group (HPEG), the temperature was raised to 65° C. after 120 minutes of reaction, and the reaction was continued for 300 minutes. After the reaction solution is cooled, stop stirring and nitrogen protection, pour the reaction solution into an excess of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com