Pharmaceutical composition containing Iloperidone and preparation method thereof
A technology of iloperidone and its composition, which is applied in the field of pharmaceutical compositions with good dissolution and its preparation, can solve the problems of particle re-aggregation and incomplete dissolution, and achieve the effects of improved dissolution, complete dissolution and improved dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
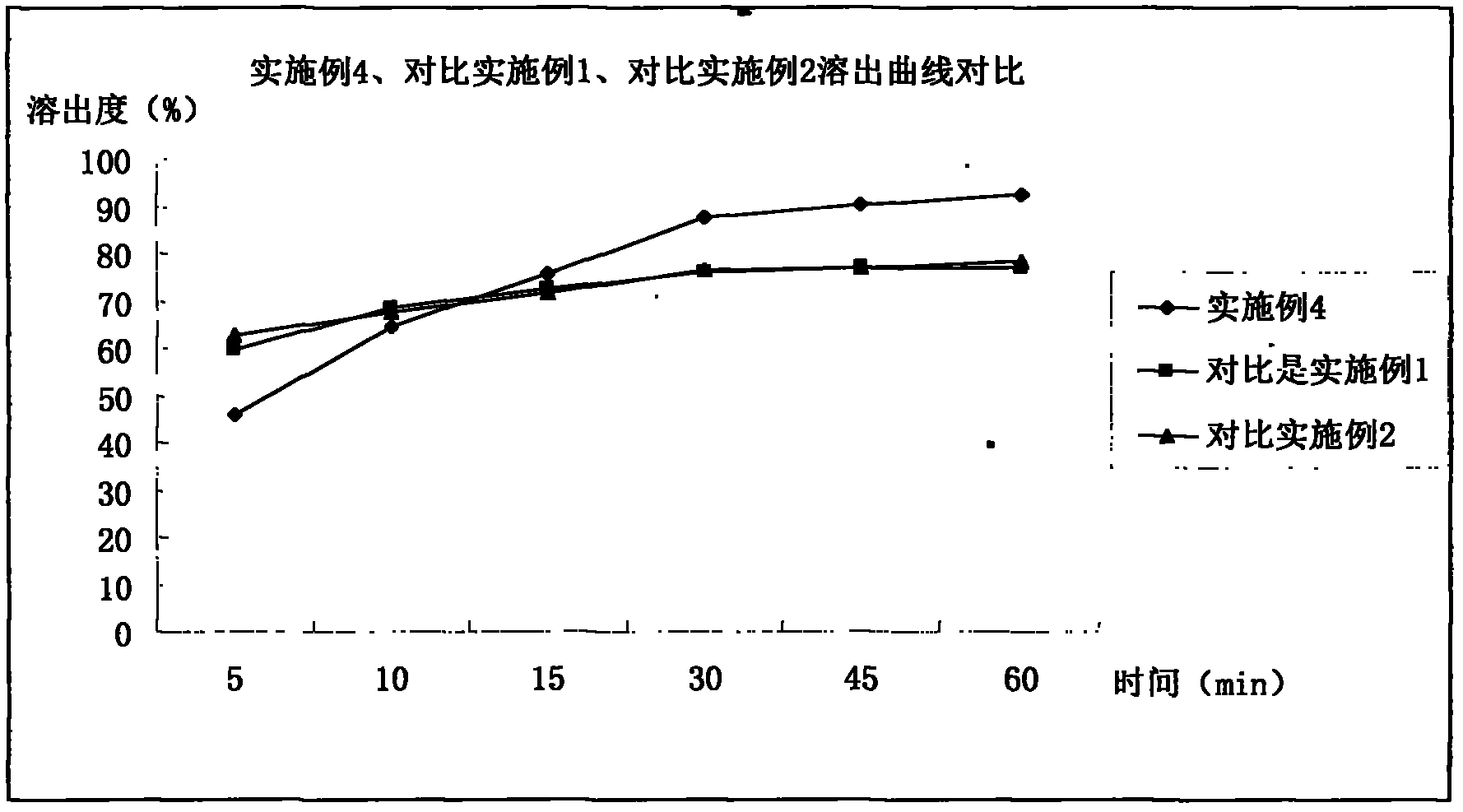
Examples
Embodiment 1
[0031]
[0032] Preparation:
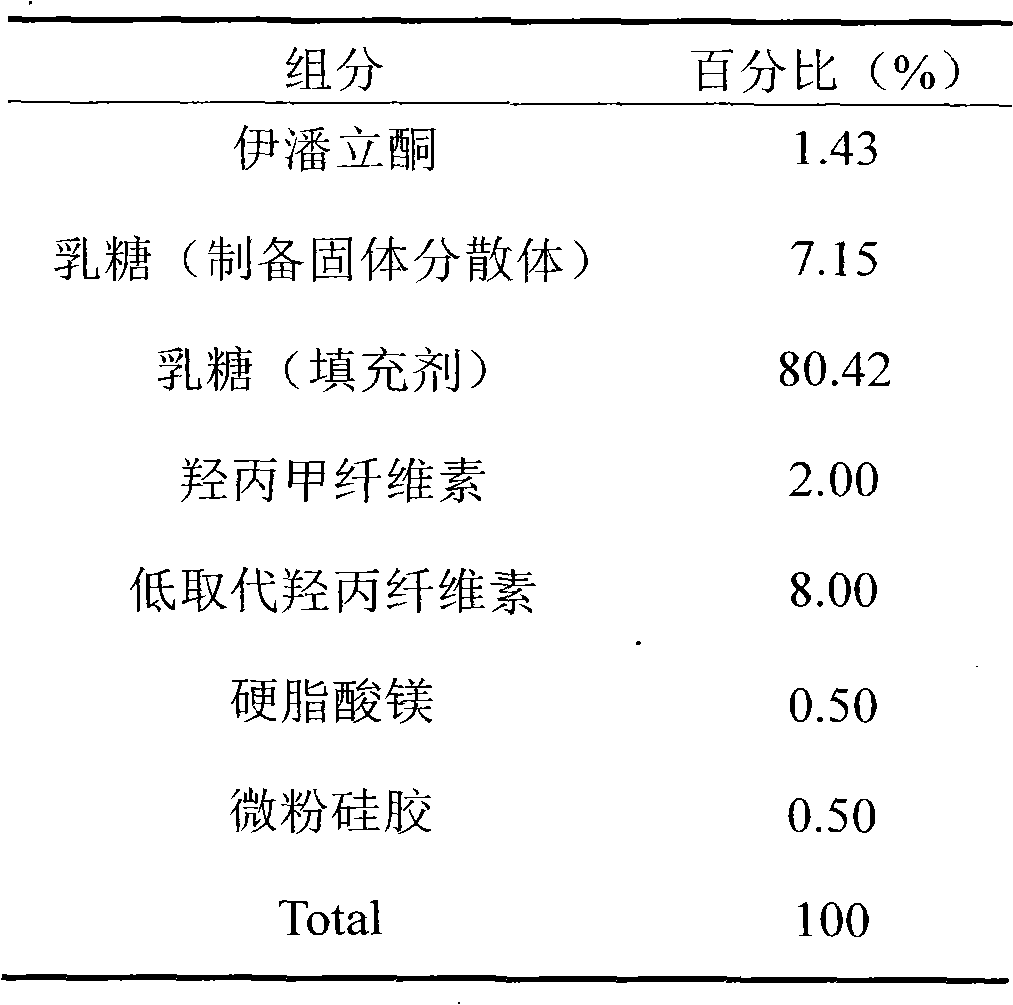
[0033] (1) Mix iloperidone and the prescribed amount of lactose uniformly, and use a jet mill to pulverize together to form a solid dispersion;
[0034] (2) Mix the above-mentioned solid dispersion with lactose, hypromellose, and low-substituted hydroxypropyl cellulose in the prescribed amount of filler, add purified water to make soft material, and granulate with 18 mesh sieve. It is ventilated and dried at a temperature of 50°C. The granules are sieved with a 24-mesh sieve, weighed, and an additional portion of low-substituted hydroxypropyl cellulose, magnesium stearate and micronized silica gel are added in proportion to mix evenly, and then compressed.
Embodiment 2
[0036]
[0037]
[0038] Preparation:
[0039] (1) Mix iloperidone and the prescribed amount of lactose uniformly, and use a jet mill to pulverize together to form a solid dispersion;
[0040] (2) Mix the above-mentioned solid dispersion with lactose, microcrystalline cellulose, hydroxypropyl cellulose and a portion of sodium carboxymethyl starch in the prescribed amount of filler, add purified water to make soft material, and use 18 mesh sieve Granulate, ventilate and dry at a temperature of 50°C, sizing with a 24-mesh sieve, weighing, adding an additional portion of sodium carboxymethyl starch and magnesium stearate in proportion, mixing uniformly, and compressing.
Embodiment 3
[0042]
[0043] Preparation:
[0044] (1) Mix iloperidone and the prescribed amount of lactose uniformly, and use a jet mill to pulverize together to form a solid dispersion;
[0045] (2) Mix the above-mentioned solid dispersion with the prescription amount of calcium hydrogen phosphate, sodium carboxymethyl cellulose, and part of croscarmellose sodium, add purified water to make soft material, and make it with 18 mesh sieve The granules are ventilated and dried at a temperature of 50°C, sieved with a 24-mesh sieve, weighed, and an additional part of croscarmellose sodium, magnesium stearate and micronized silica gel are added in proportion, mixed evenly, and compressed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com