Cells producing antibody compositions
A technology of antibody composition and cells, applied in the direction of fusion cells, antibody mimics/scaffolds, antibodies, etc., can solve problems such as determining effector functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0614] Generation of anti-ganglioside GD3 human chimeric antibody:
[0615] 1. Construct a tandem expression vector pChiLHGM4 for anti-ganglioside GD3 human chimeric antibody
[0616] Using a DNA ligation kit (manufactured by Takara Shuzo), the plasmid pChi641LGM40 was constructed by ligating an approximately 4.03 kb fragment containing the L-chain cDNA and an approximately 3.40 kb fragment containing the G418 resistance gene and splicing signal, the former by using the restriction enzyme MluI (Takara Shuzo manufactured) and SalI (manufactured by Takara Shuzo) digested pChi641LGM4 of the L chain expression vector, anti-ganglioside GD3 human chimeric antibody (hereinafter referred to as "anti-GD3 chimeric antibody") [J.Immunol.Methods, 167 , 271 (1994)], the latter was obtained by digesting the animal cell expression vector pAGE107 using restriction enzymes MluI (manufactured by Takara Shuzo) and SalI (manufactured by Takara Shuzo) [Cytotechnology, 3, 133 (1990)], and then tra...
Embodiment 2
[0646] Activity assessment of anti-GD3 chimeric antibody:
[0647] 1. Binding activity of anti-GD3 chimeric antibody to GD3 (ELISA)
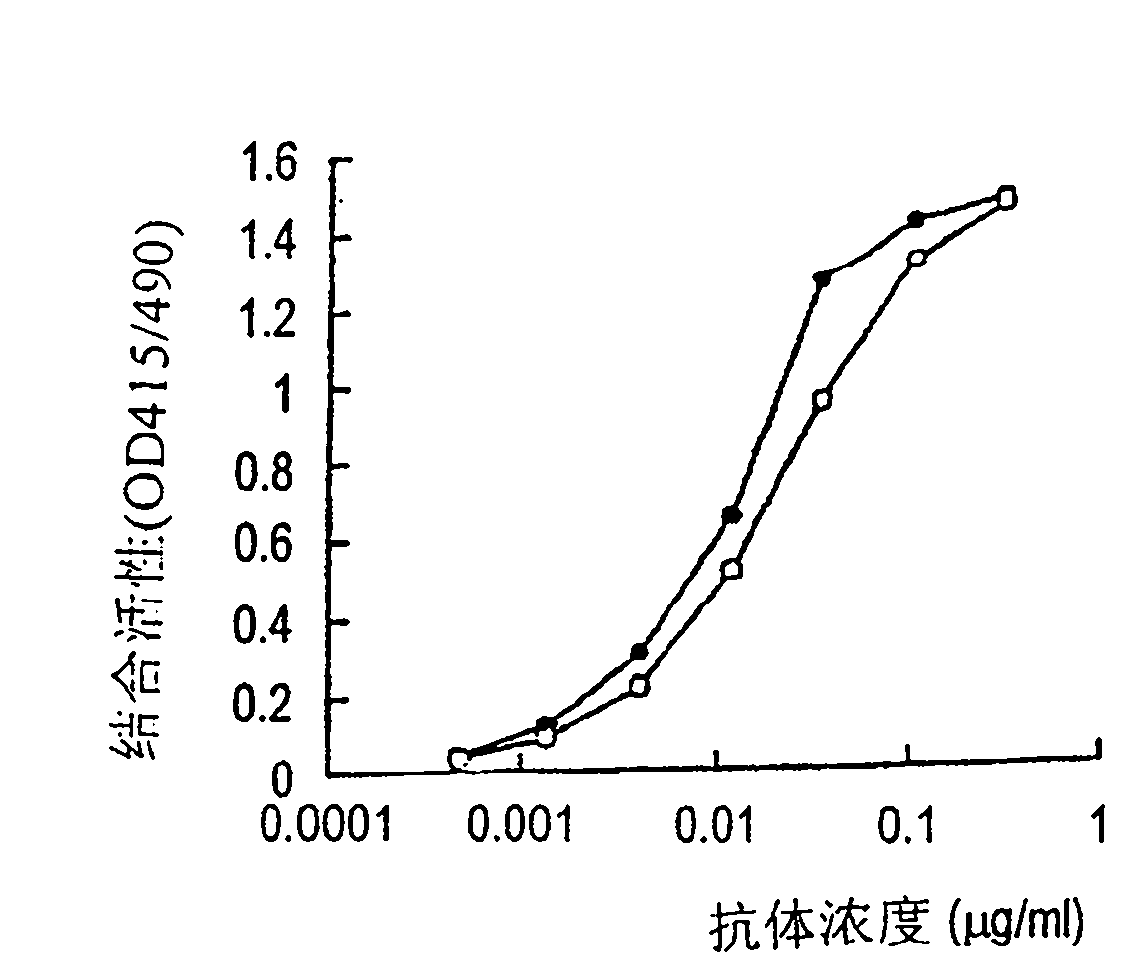
[0648] The binding activity of the five purified anti-GD3 chimeric antibodies to GD3 (manufactured by Snow Brand Milk Products) in Item 4 of Example 1 was determined by the ELISA method shown in Item 3 in Example 1. figure 2 The detection results of the binding activity determined by changing the concentration of the added anti-GD3 chimeric antibody are shown. as in figure 2 As shown in , five anti-GD3 chimeric antibodies showed almost the same binding activity as GD3. This result shows that the antigen-binding activity of these antibodies is invariant independent of the antibody-producing cells and culture method. It was also shown from the comparison of the NSO-GD3 chimeric antibody (302) with the NSO-GD3 chimeric antibody (GIT) that the antigen-binding activity was unchanged regardless of the medium used in the culture.
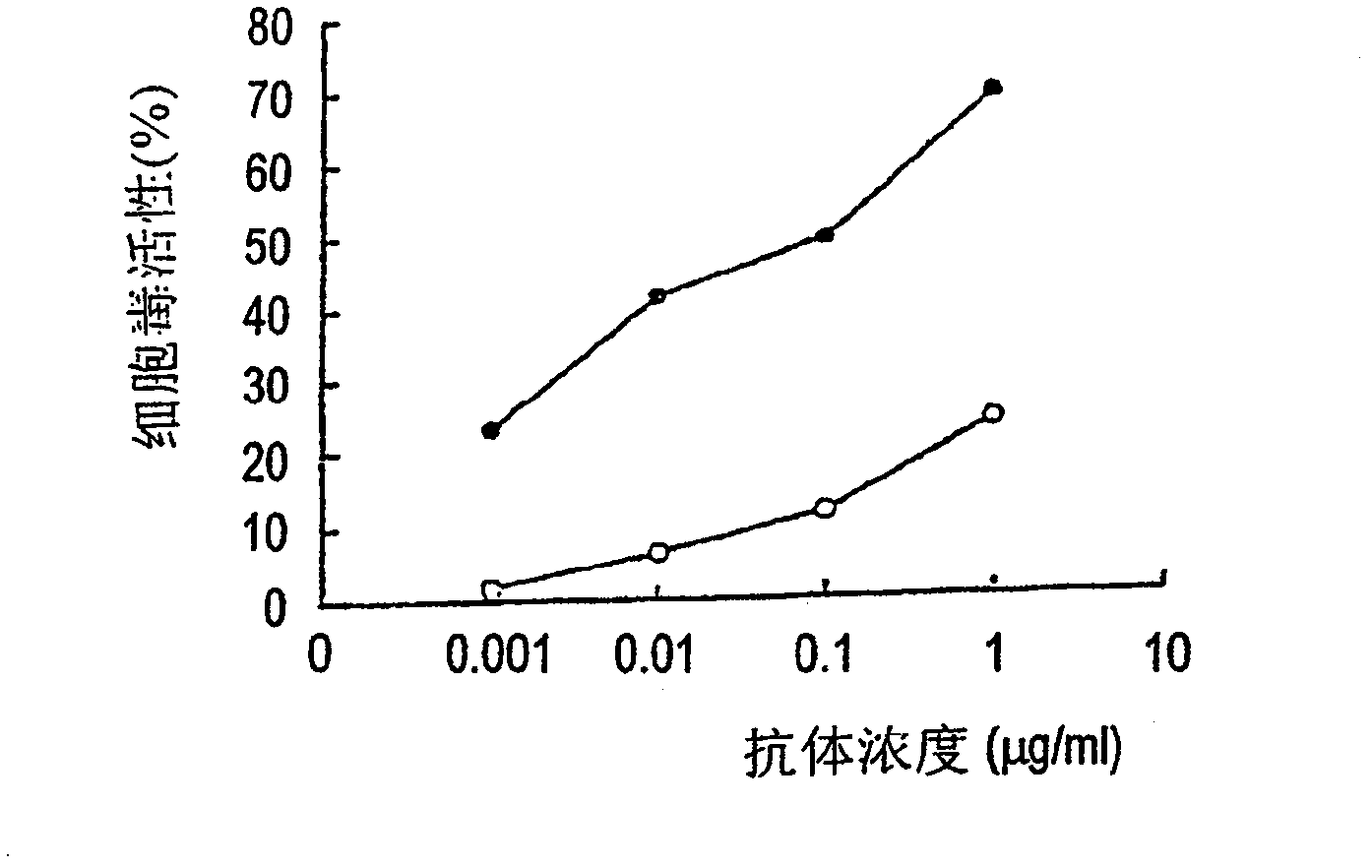
[0649] 2. In v...
Embodiment 3
[0660] Preparation of anti-human interleukin-5 receptor alpha chain human CDR-grafted antibody:
[0661] 1. Preparation of cells that stably produce anti-human interleukin-5 receptor α chain human CDR-grafted antibody
[0662] (1) Rat myeloma YB2 / 0 cells were used to prepare antibody-producing cells
[0663] Using the anti-human interleukin 5 receptor α chain human CDR-grafted antibody (hereinafter referred to as "anti-hIL-5Rα CDR-grafted antibody") expression vector, pKANTEX1259HV3LV0 described in WO97 / 10354, was prepared as follows capable of stably producing anti-hIL-5Rα CDR Cells transplanted with antibodies.
[0664] After transplanting 5 μg of anti-hIL-5Rα CDR into the antibody expression vector pKANTEX1259HV3LV0 by electroporation [Cytotechnology, 3, 133 (1990)] introducing 4×10 6 After inoculation into rat myeloma YB2 / 0 cells, the cells were suspended in 40 ml of RPMI640-FBS (10), and distributed to 96-well plates (manufactured by Sumitomo Bakelite) at 200 µl / well. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com