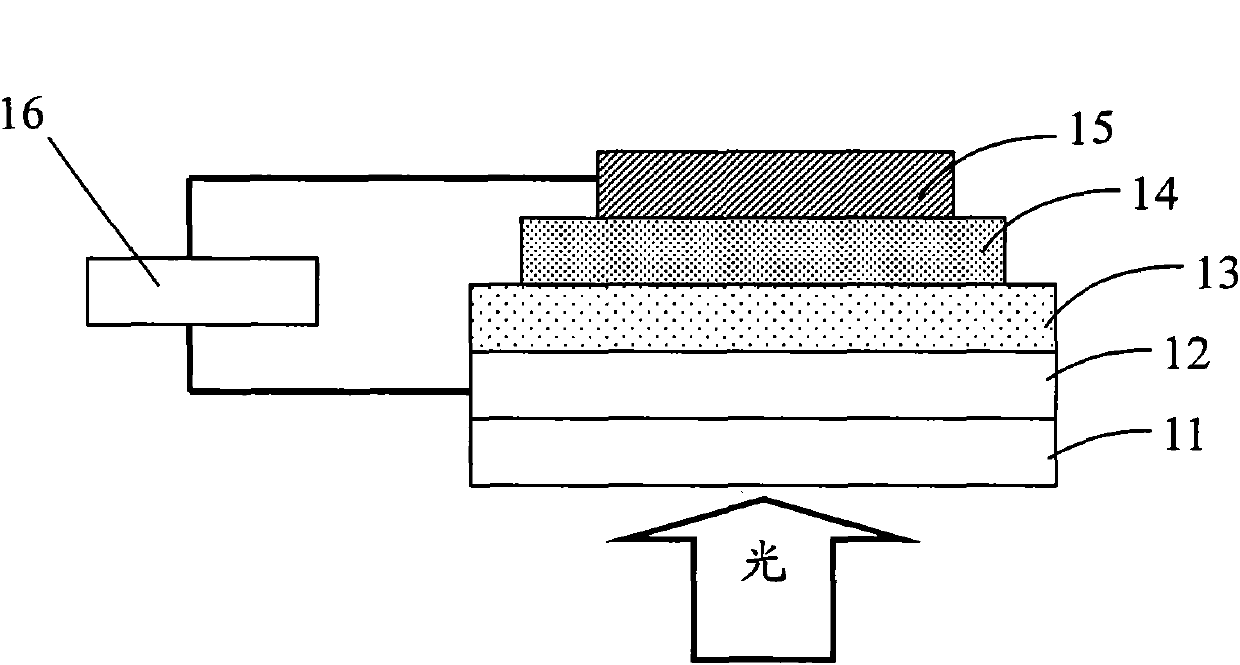
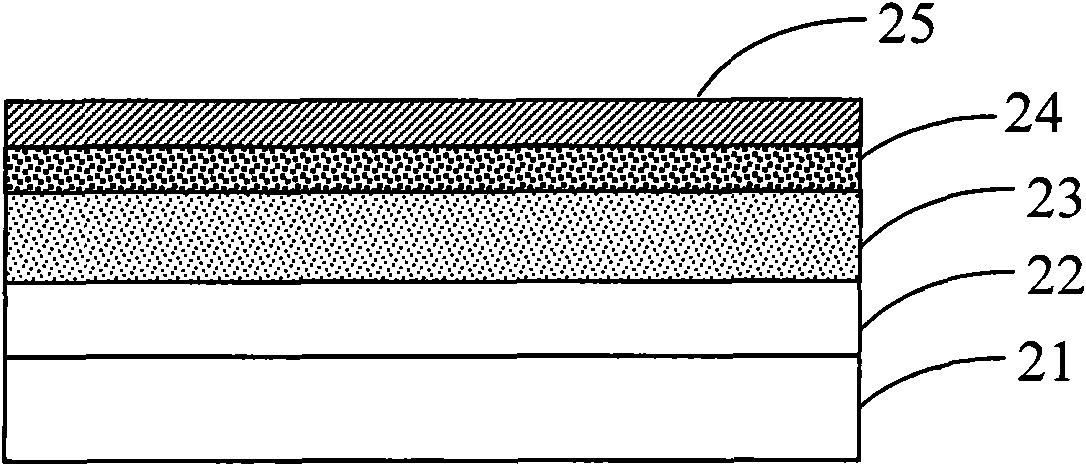
Copolymer comprising silafluorene and thiophene pyrroledione units and preparation method as well as application thereof
A technology of thienopyrrole diketone and copolymer, which is applied in the fields of electrical components, active region structure, semiconductor/solid-state device manufacturing, etc., and can solve the problem of low photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
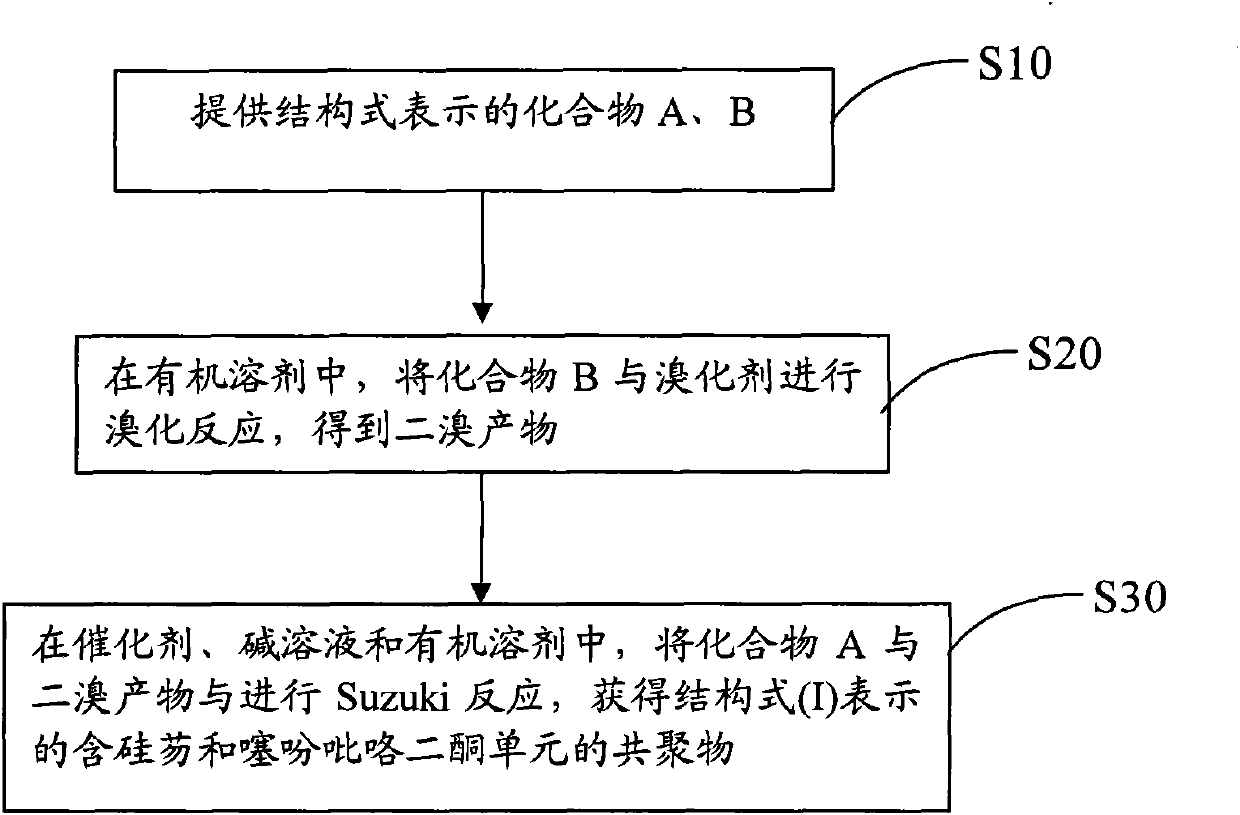
[0029] see figure 1 , the preparation method of the above-mentioned copolymer containing silicon fluorene and thienylpyrrole diketone unit comprises the steps:
[0030] S10, providing compounds A and B represented by the following structural formula,
[0031] Where: R 1 , R 2 , R 3 from C 1 -C 20 the alkyl group;
[0032] S20, in an organic solvent, the compound B is brominated with a brominating agent to obtain a dibrominated product;
[0033] S30, in catalyst, alkali solution and organic solvent, carry out Suzuki reaction with compound A and dibromo product, obtain the copolymer containing silicon fluorene and thienopyrrole dione unit represented by following structural formula (I):
[0034] In the formula, n is an integer of 1-100.
[0035] In step S10, compounds A and B can be directly purchased from the market or prepared by existing synthetic methods. Among them, the structures of compounds A and B are basically the same as those described above for the copo...
Embodiment 1
[0053] The copolymer (I 1 ), R 1 , R 2 , R 3 Both are methyl, and its structural formula is as follows:
[0054]
[0055] In the above formula, n=20.
[0056] The copolymer of the present embodiment (I 1 ) preparation comprises the following specific steps:
[0057] 1. The preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dimethylsilfluorene, compound A A concrete example, its structural formula is as follows:
[0058]
[0059]The specific preparation process is as follows: at -100°C under nitrogen, add 40.00mL (1.00M) n-butyllithium solution to a solution containing 3.68g 2,7-dibromo-9,9-dimethylsilafluorene and 120mL In the tetrahydrofuran reactor, after stirring for 2 hours, slowly add 4.33mL 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane dropwise to restore to Stirring was continued for 48 hours at room temperature. After the reaction, the reaction solution was poured into water, extracted with ether, dried over anhydrous magnesium sul...
Embodiment 2
[0073] The copolymer (I 1 ), R 1 , R 2 , R 3 Both are C 8 h 17 , its structural formula is as follows:
[0074] In the above formula, n=58.
[0075] The copolymer of the present embodiment (I 2 ) preparation comprises the following specific steps:
[0076] 1. The preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dioctylsilfluorene, compound A A concrete example, its structural formula is as follows:
[0077]
[0078] The specific preparation process is as follows: Add 10.00mL (2.00M) n-butyllithium solution with a syringe to 5.65g 2,7-dibromo-9,9-dioctylsilafluorene at -78°C under nitrogen gas and 100.00mL tetrahydrofuran in a two-necked flask, after stirring for 2 hours, slowly add 4.90mL 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane , returned to room temperature, and continued to stir for 33 hours. After the reaction, the reaction solution was poured into water, extracted with ether, dried over anhydrous magnesium sulfate, rotary evap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com