Methylprednisolone sodium succinate freeze-dried powder injection and preparation method thereof
A technology of methylprednisolone sodium succinate and freeze-dried powder injection, which is applied in the field of methylprednisolone sodium succinate composition, and can solve the problems of low production efficiency and freeze-drying time of more than 30 hours
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
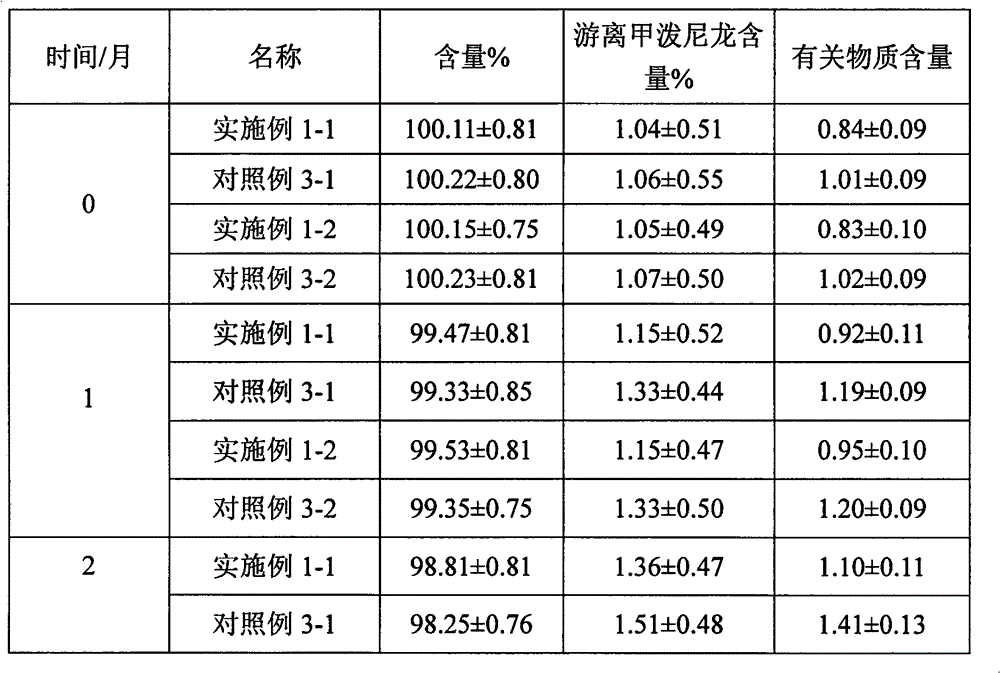
Examples
Embodiment 1
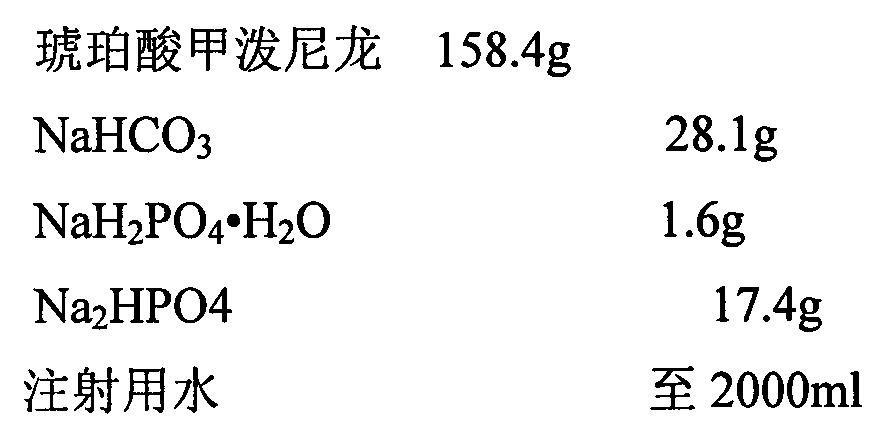
[0019]
[0020] The preparation method is as follows:
[0021] 1. Weigh the prescription amount of NaHCO 3 , add 200ml of water for injection to dissolve, set aside.
[0022] 2. Weigh the prescription amount NaH 2 PO 4 ·H 2 O, add 200ml of water for injection to dissolve, set aside.
[0023] 3. Weigh the prescription amount Na 2 HPO 4 , add 200ml of water for injection to dissolve, set aside.
[0024] 4. Weigh the main drug of the prescribed amount, add 1200ml water for injection to stir into a suspension, and slowly add NaHCO under constant stirring 3 solution, heated to 40-50°C in a water bath and stirred, adding NaH 2 PO 4 ·H 2 O solution, followed by Na 2 HPO 4 Adjust the pH value of the solution, make up water for injection to 2000ml, keep warm in a water bath until the solution is clear, and circulate and filter the whole amount.
[0025] 5. Filter the medicinal solution with a 0.45 μm microporous membrane, and finally fine filter with a 0.22 μm microporo...
Embodiment 2
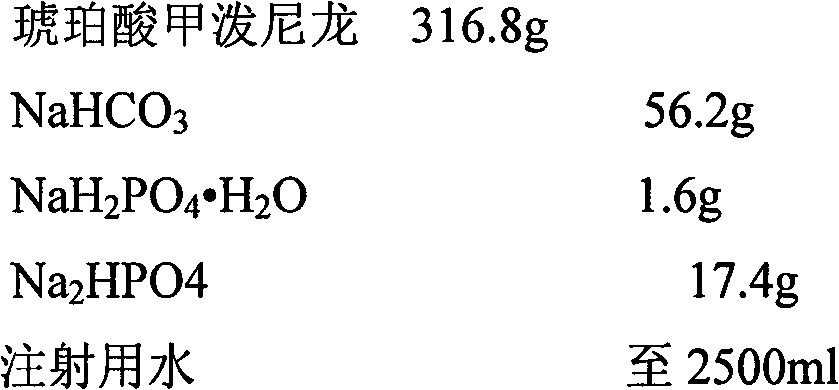
[0033]
[0034] The preparation method is as follows:
[0035] 1. Weigh the prescription amount of NaHCO 3 , add 200ml of water for injection to dissolve, set aside.
[0036] 2. Weigh the prescription amount NaH 2 PO 4 ·H 2 O, add 200ml of water for injection to dissolve, set aside.
[0037] 3. Weigh the prescription amount Na 2 HPO 4 , add 200ml of water for injection to dissolve, set aside.
[0038] 4. Weigh the main drug of the prescribed amount, add 1500ml water for injection to stir into a suspension, and slowly add NaHCO under continuous stirring 3 solution, heated to 40-50°C in a water bath and stirred, adding NaH 2 PO 4 ·H 2 O solution, followed by Na 2 HPO 4 Adjust the pH value of the solution, make up water for injection to 2500ml, keep warm in a water bath until the solution is clear, and circulate and filter the whole amount.
[0039]5. Filter the medicinal solution with a 0.45 μm microporous membrane, and finally fine filter with a 0.22 μm micropor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com