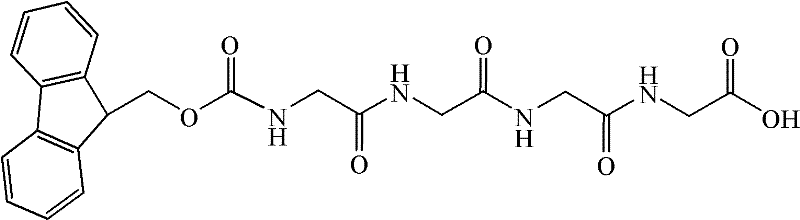
Preparation method for bivalirudin
A technology of bivalirudin and resin, which is applied in the field of polypeptide drug preparation, can solve problems such as difficult complete purification, reduced product purity, and inability to effectively improve the total yield of the product, and achieves warm reaction conditions, simple reaction operation, and wide application Effects on value and application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The preparation of embodiment 1Fmoc-Leu-Wang resin
[0059] Take wang resin 500g (substitution value 1.0mmol / g), swell with 5L N,N-dimethylamide (DMF) for 30 minutes, add Fmoc-Leu-OH 353g (1.0mol), stir for 30 minutes and then add 155ml DIC (1.0mol), 135gHOBt (1.0mol), 6.1g (0.05mol) DMAP, stirred and reacted at room temperature for 18 hours, and after filtration, the resin was washed 3 times with DMF, dichloromethane (DCM), methanol, and dried under reduced pressure to obtain Fmoc-Leu-Waug resin 651g, esterification yield 95.6%.
Embodiment 2
[0060] Embodiment 2 Fmoc-Leu-Wang resin de-Fmoc protection obtains H-Leu-Wang resin
[0061] Take the above Fmoc-Leu-Wang resin, swell with 5L 20% piperidine (PIP) / NN-dimethylamide (DMF) solution for 10 minutes, add 5L 20% PIP / DMF solution after filtration, and stir at room temperature for 25 After filtering, the resin was washed three times with DMF, DCM and methanol, and dried under reduced pressure to obtain the preparation of H-Leu-Wang resin.
Embodiment 3
[0062] The preparation of embodiment 3Fmoc-Leu-2-Cl-Trt resin
[0063] Take 500g of 2-Cl-Trt-Cl resin (substitution value 1.0mmol / g), swell with 5LN, N-dimethylamide (DMF) for 30 minutes, add 353g (1.0mol) of Fmoc-Leu-OH, and stir Add 260ml DIEA (1.5mol) after 30 minutes, stir at room temperature for 3 hours, filter and wash the resin 3 times with DMF, DCM, methanol, and dry under reduced pressure to obtain 655g of Fmoc-Leu-2-Cl-Trt resin, esterification Yield 98.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com