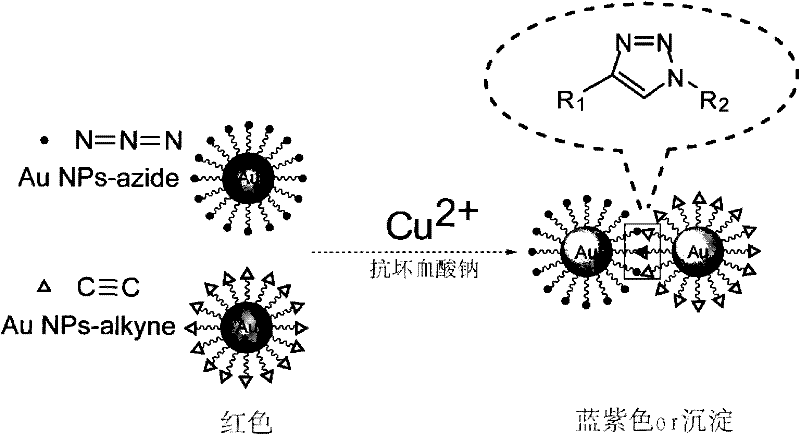
Method, kit and application of copper oxide nanoparticle-labeled antibody
A nanoparticle and labeled antibody technology, applied in the field of immunoassay and diagnosis, can solve the problems affecting the detection limit of the test substance, environmental pollution and health, complex methods, etc., and achieve the effects of low cost, good stability and simple operation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
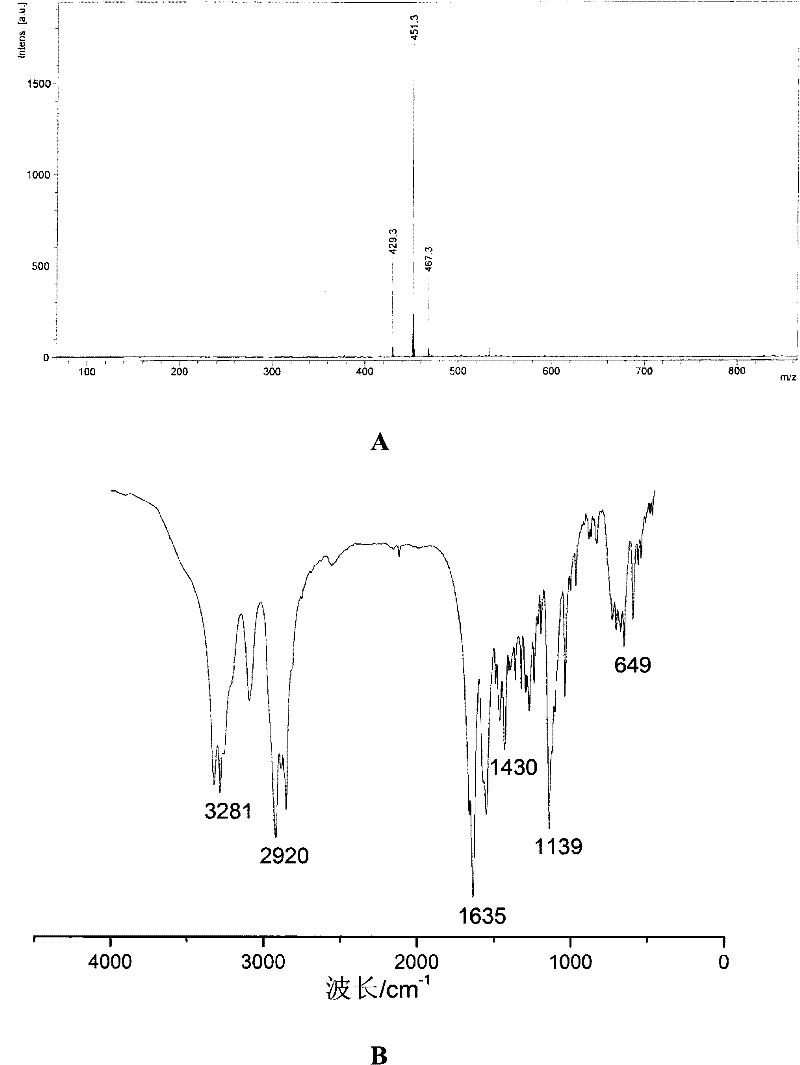
[0056] Embodiment 1: the synthesis of compound 1
[0057] Compound 1
[0058] step:
[0059] 1. Vacuumize a 100mL two-neck flask (19#) and fill it with nitrogen.
[0060] 2. Add 50 mL of toluene to the flask under nitrogen protection, and dissolve triphenylchloromethane (4.60 g, 16.5 mmol) and N, N-diisopropylethylamine (DIEA, 4.20 g, 33.0 mmol) in the toluene Inside, 11-mercaptoundecanoic acid (3.00 g, 13.8 mmol) was added under magnetic stirring, and reacted at room temperature for 5 hours under nitrogen protection.
[0061] 3. After the reaction is complete, distill the solvent off under reduced pressure, add 50 mL of dichloromethane to the residual product, and after fully dissolving, wash with saturated brine (3×100 mL) three times, then dry the dichloromethane solution with anhydrous sodium sulfate , let stand overnight.
[0062] 4. Remove the desiccant anhydrous sodium sulfate by filtration, distill the filtrate under reduced pressure, and concentrate to obtain ...
Embodiment 2
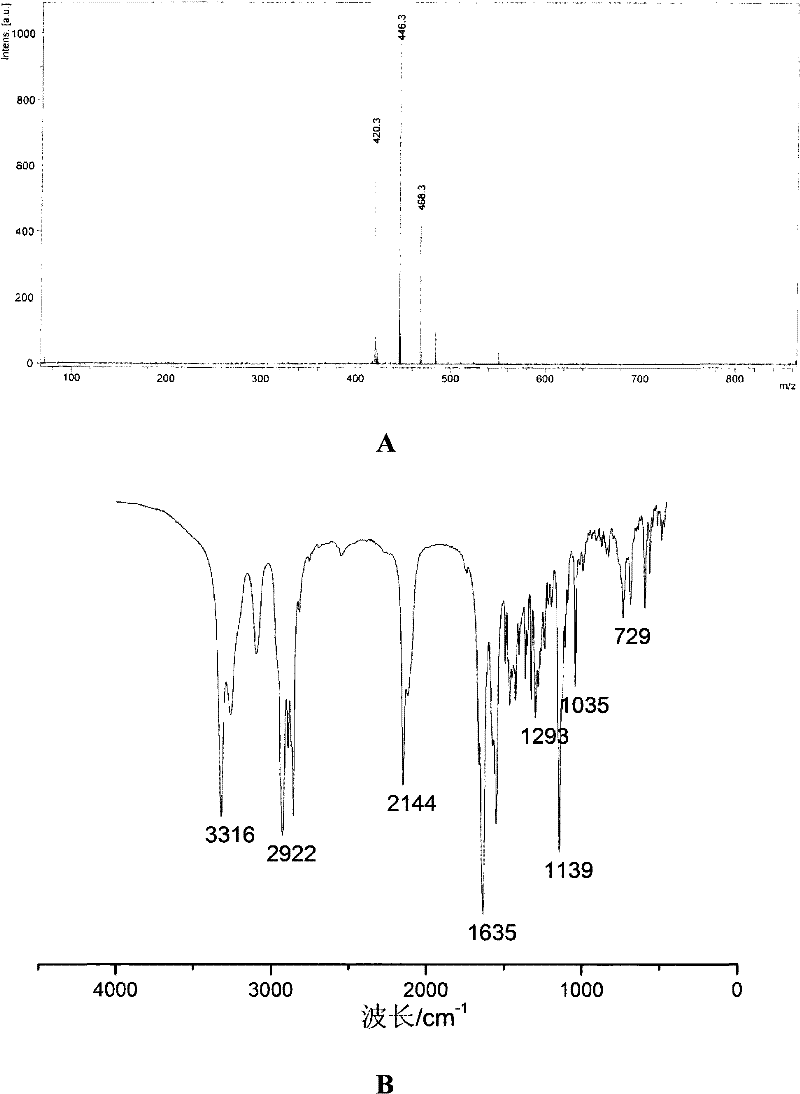
[0063] Embodiment 2: the synthesis of compound 2
[0064] Compound 2
[0065] step:
[0066] 1. Add compound 1 (1.50g, 3.3mmol) in a 50mL single-necked flask (19#), EDC-HCl (0.69g, 3.6mmol) catalytic amount of DMAP, add 25mL of anhydrous dichloromethane, magnetically stir to make After dissolution, N-hydroxysuccinimide (NHS, 0.45 g, 3.9 mmol) was finally added to the mixture.
[0067] 2. The mixed solution was stirred at 5° C. for one hour, and then reacted at room temperature for 24 hours.
[0068] 3. After the reaction, the reaction solution was diluted with 25 mL of dichloromethane, washed three times with saturated brine (3×50 mL), dried the organic phase with anhydrous sodium sulfate, and concentrated to obtain compound 2 (1.80 g, 3.23 mmol). is the activated ester of compound 1. The yield was 99%.
Embodiment 3
[0069] Embodiment 3: the synthesis of compound 3
[0070]
[0071] step:
[0072] 1. Add NH 2 C 2 h 4 OC 2 h 4 OC 2 h 4 NH 2 (8mL, 55mmol) was dissolved in anhydrous dichloromethane, and slowly added dropwise to the mixed solution 10mL of compound 2 (1.80g, 3.23mmol) in dichloromethane under stirring, after the addition was complete, react at room temperature overnight.
[0073] 2. Dilute the reaction solution with 50 mL of dichloromethane, wash the organic phase with saturated brine (3×50 mL) three times, dry the organic phase with anhydrous sodium sulfate, and concentrate under reduced pressure to obtain the crude compound 3.
[0074] 3. Dissolve the crude product with a little dichloromethane and apply it as a sample, then separate by column chromatography, the eluent is chloroform: methanol: ammonia water = 20:1:0.05, the pure product is a colorless oily liquid, a total of 1.78g (3.0mmol ), the yield was 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com