A kind of lamivudine tablet and preparation method thereof
A lamivudine tablet, lamivudine technology, applied in the direction of pharmaceutical formulations, antiviral agents, medical preparations of non-active ingredients, etc., can solve problems such as unstable quality, and achieve good fluidity and disintegration Effects of short duration and low friability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0043] Experimental Example 1 Comparative Screening Experiment
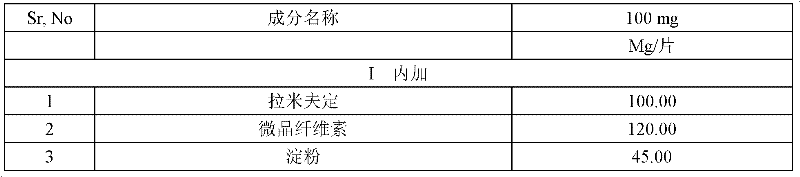
[0044] 1.1 General ratio and preparation method
[0045] Table 1 uses general proportioning and preparation method gained lamivudine tablet experimental data
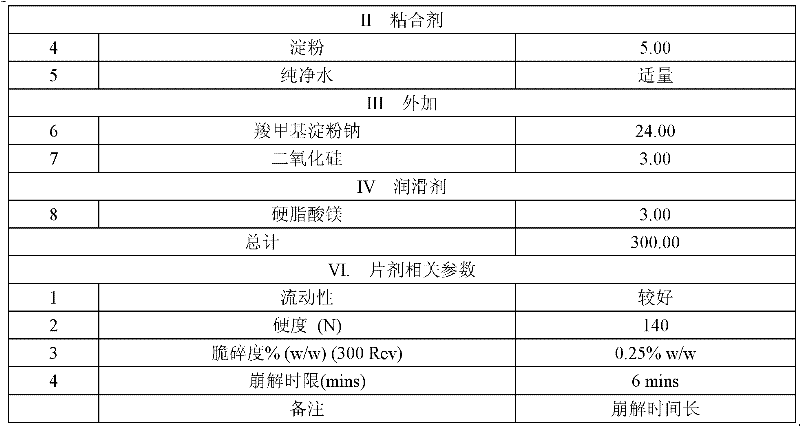
[0046]
[0047]
[0048] Results: The disintegration time limit of the tablet was too high, and the tablet disintegrated layer by layer and could not be finely dispersed, which further affected the final dissolution rate of the ingredients.
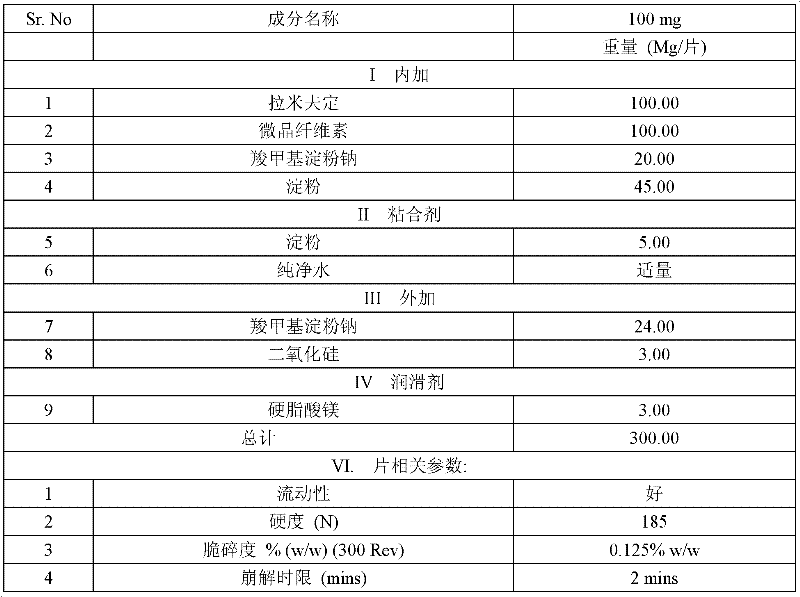
[0049] 1.2 Improve disintegration by adding sodium carboxymethyl starch inside and outside. The results are shown in Table 2.
[0050] Table 2 Lamivudine tablet experimental data of the present invention (made by embodiment 1)
[0051]
[0052]
[0053] The above experimental data show that the lamivudine tablet of the present invention is well dispersed in the dissolution medium, swells uniformly, and is dispersed into fine particles.
[0054] 1.3 According to the above ratio, a batch of 150,000 pieces ...
experiment example 2
[0059] Experimental Example 2 Accelerated comparative experiment
[0060] Lamivudine tablet of the present invention (made by embodiment 1) compares with listing lamivudine tablet stability
[0061] 2.1 Storage conditions
[0062] Store at a temperature of 40°C±2°C and a relative humidity of 75%±5%, take samples at 0, 1, 3, and 6 months, and check according to the draft quality standard for lamivudine tablets.
[0063] 2.2 Inspection items:
[0064] (1) Properties: visual inspection.
[0065] (2) Dissolution rate: in 30 minutes, the dissolution amount is not less than 80% of the labeled amount.
[0066] (3) Related substances: carboxylic acid impurities with a relative retention time of 0.4 do not exceed 0.3%; salicylic acid does not exceed 0.1%; impurities with a relative retention time of 0.9 do not exceed 0.2%; total impurities do not exceed 0.6%.
[0067] (4) Content: 90% to 110%.
[0068] The results are shown in the table below:
[0069] Table 4 090502 batches of l...
experiment example 3
[0074] Experimental Example 3 Comparative Experiment of Influencing Factors
[0075] This experiment is carried out under more severe conditions than the accelerated experiment. The purpose is to explore the inherent stability of the drug, understand the factors affecting its stability, possible degradation pathways and degradation products, and provide scientific basis for the preparation process, packaging, storage conditions and the establishment of analysis methods for degradation products.
[0076] Experimental conditions: placed under the conditions of high temperature (60°C), high humidity (92.5% RH), and light (4500Lx±500Lx), sampling regularly, and measuring various indicators.
[0077] Table 6 090502 batches of lamivudine tablet influence factor test results of the present invention (high temperature 60 ℃)
[0078]
[0079] Table 7 090502 batches of lamivudine tablet influencing factor test results (light) of the present invention
[0080]
[0081] Table 8 09...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com