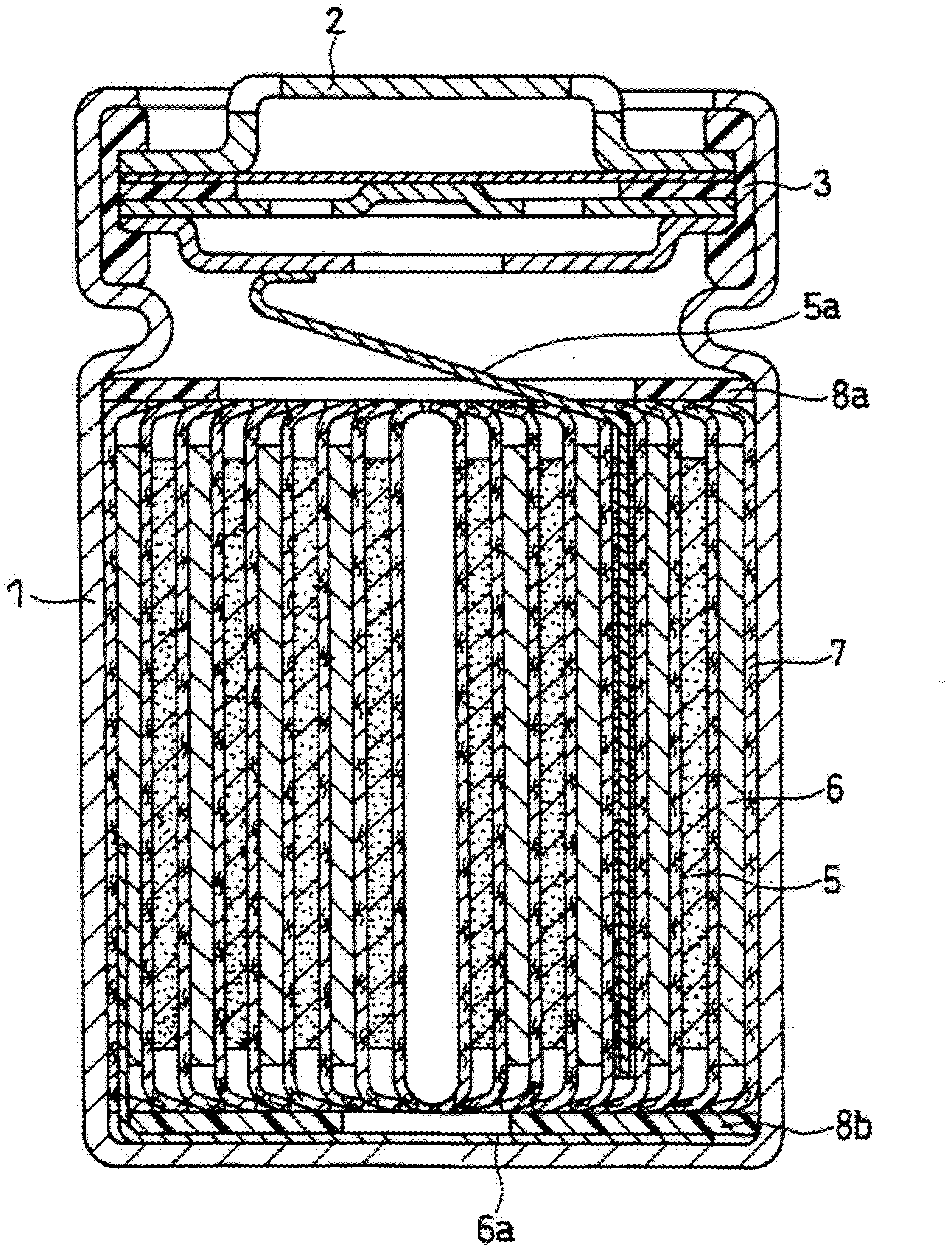
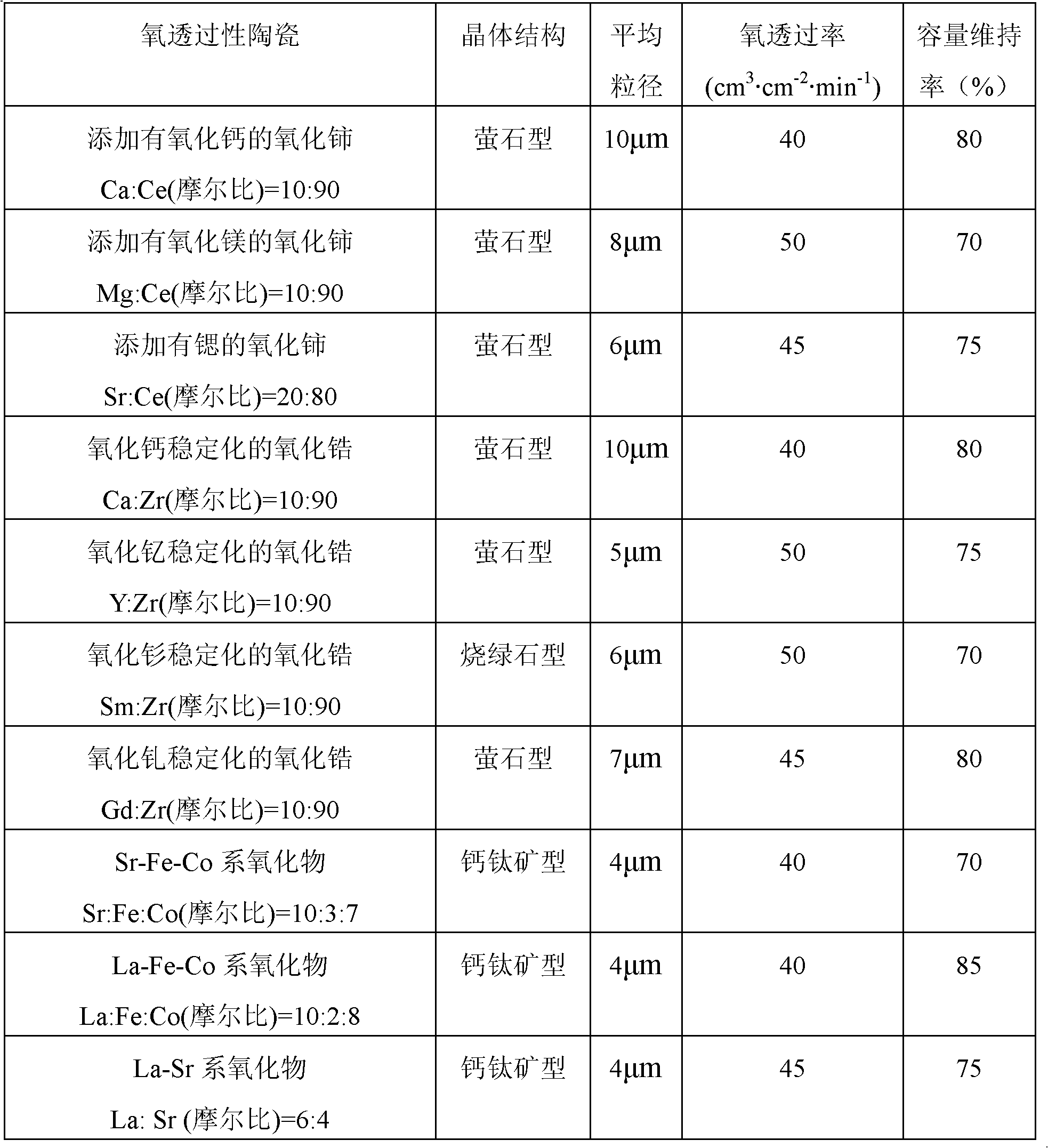
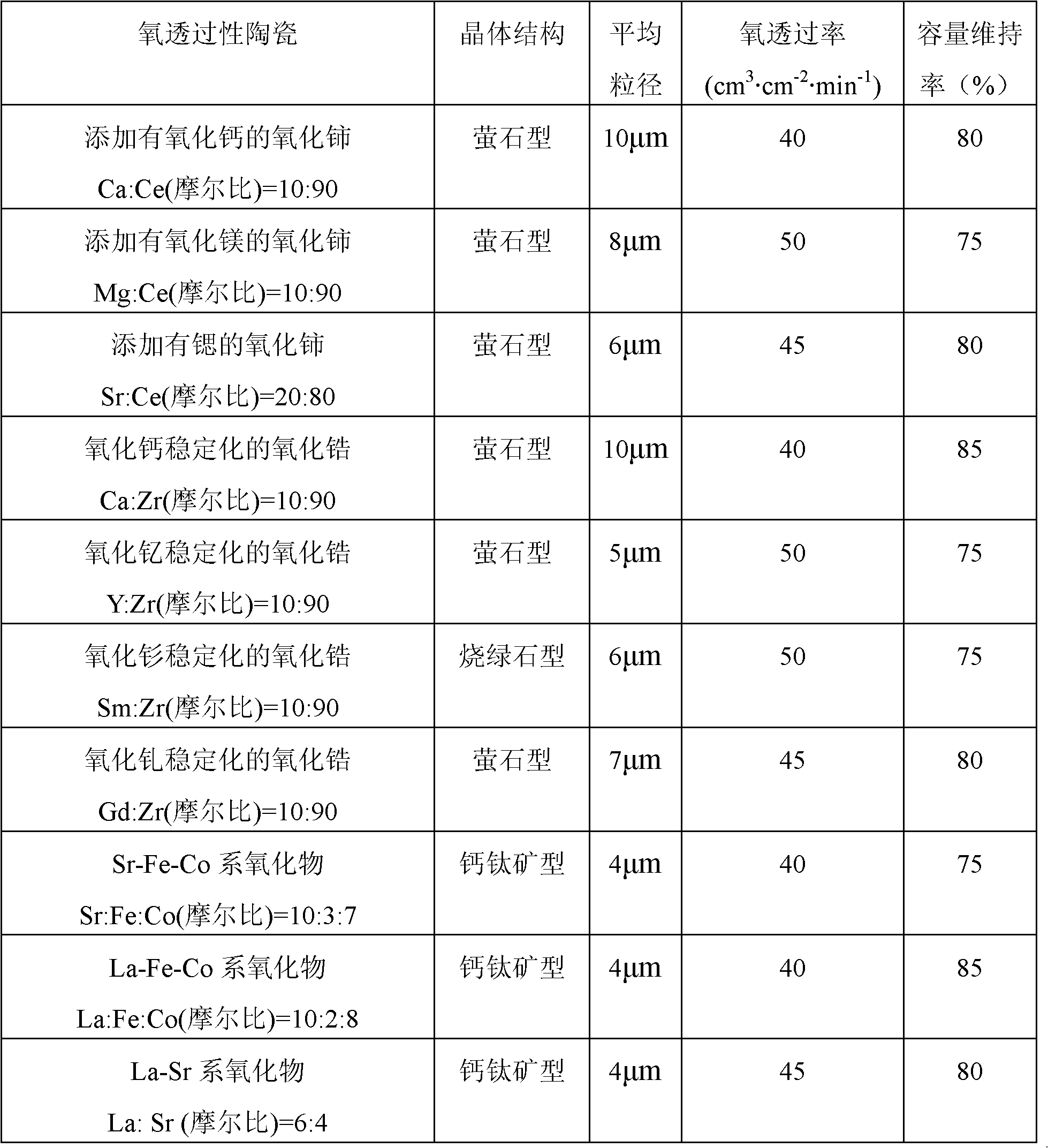
Positive electrode active material for nonaqueous electrolyte secondary battery and method for producing same
A positive electrode active material and non-aqueous electrolyte technology, applied in battery electrodes, circuits, nickel compounds, etc., can solve the problem of high process costs and achieve the effect of suppressing the formation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The production method of the hydroxide is not particularly limited. However, from the viewpoint of easy synthesis of the lithium-nickel composite oxide, it is desirable to incorporate the element L into the crystal structure of the nickel-containing hydroxide, and it is desirable to form a solid solution of nickel and the element L. Such a solid solution can be synthesized by, for example, coprecipitation. In the co-precipitation method, in order not to aggregate elements that are more easily oxidized than Ni, it is preferable to precipitate the hydroxide in a reducing atmosphere.
[0042] In the case of the co-precipitation method, for example, a method of preparing an aqueous solution of a raw material salt mixture containing nickel and the element L in a predetermined molar ratio and adding a base thereto to obtain a co-precipitated hydroxide can be mentioned. At this time, the pH in the aqueous solution is preferably 7-14. Moreover, it is preferable that water tem...
Embodiment 1
[0081] (i) Synthesis of nickel-containing hydroxide
[0082] 3.2 kg of a mixture of nickel sulfate and cobalt sulfate obtained by mixing Ni atoms to Co atoms in a molar ratio of 80:20 was dissolved in 10 L of water to obtain a raw material solution. 400 g of sodium hydroxide was added to the raw material solution to generate a precipitate. The precipitate was sufficiently washed with water and dried to obtain a co-precipitated hydroxide.
[0083] (ii) Addition of oxygen-permeable ceramics
[0084] A solution in which calcium sulfate and zirconium sulfate were dissolved in ion-exchanged water at a molar ratio of 3:17 was prepared. The co-precipitated hydroxide (Ni 0.8 co 0.2 (OH) 2 ) was dispersed in 3 L of the solution, stirred at 25° C. for 3 hours, and then dried at 100° C. for 2 hours to obtain an intermediate of a composite oxide. The added amount of the oxygen-permeable ceramic precursor determined from the weight increase rate was 0.5 parts by weight per 100 parts ...
Embodiment 2
[0108] In the synthesis process of hydroxide, the molar ratio of Ni atoms to Co atoms is set to 60:40 to synthesize Ni 0.6 co 0.4 (OH) 2 , except for using it, a battery was produced in the same manner as in Example 1, and the capacity retention rate was obtained in the same manner. The capacity maintenance rate was 75%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com