Chemical preparation method of norfloxacin
A norfloxacin and chemical technology, applied in the field of chemical preparation of norfloxacin, can solve the problems of poor selectivity, no economic value, low product conversion rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
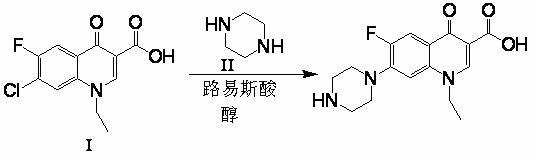
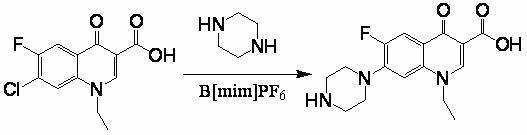
[0033] Example 1 , drop into 30g (0.111mol) 1-ethyl-6-fluoro-7-chloro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, 60g piperazine (0.697mol) in four-necked flask mol), 75g of isoamyl alcohol, 1.5g of aluminum trichloride (0.0112mol), heated to 100 ° C for 12 hours, then added 4.5g of sodium hydroxide (0.113mol), recovered to dryness under reduced pressure, and added to the residue 150g water, 4.5g sodium hydroxide (0.113mol), 0.1g activated carbon, heat up to 90°C for 30 minutes, filter, adjust the pH of the obtained filtrate to neutral, cool to room temperature and suction filter, and dry the filter cake at 100°C 31.6 g of the product was obtained, and the liquid phase detection content was 99.5%, of which the content of the 6-position fluorine-substituted isomer was 0.1%. Yield 88.9%.
[0034] Example 2 , drop into 30g (0.111mol) 1-ethyl-6-fluoro-7-chloro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, 27g piperazine (0.313 mol), 13g methanol, 30g aluminum trichloride...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com