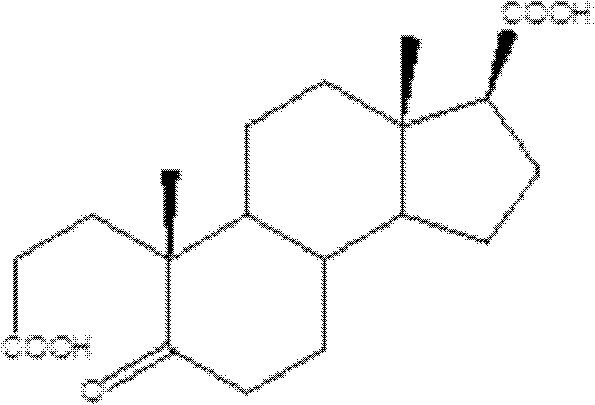
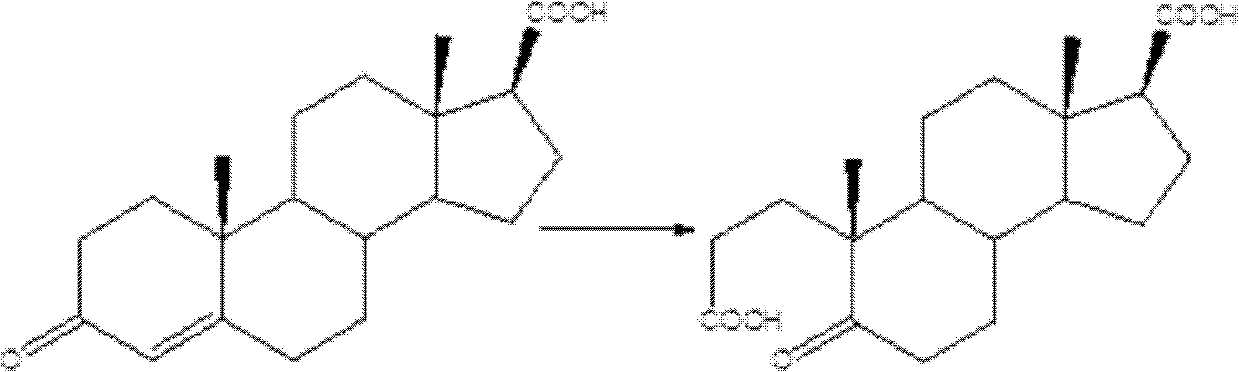
Synthetic method of A-nor-3,5-cracking-androstane-5-ketone-3,17 beta-diacid
A technology of androstene and diacid, which is applied in the preparation of ketene/polyketene, organic chemistry, etc., can solve the problems of unsuitability for large-scale industrial production, pollution of heavy metal ions, and difficulty in separation and purification, and achieve easy industrial implementation and three wastes ”Effect of reduced emission and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of A-carbo-3,5-cleavage-androst-5-one-3,17β-diacid:
[0024] Add 3-carbonyl-4-androstene-17-carboxylic acid (31.64g; Fw: 316.43 ; 100mmol), then add 100mL acetone and hydrogen peroxide (30wt%H 2 o 2 , 100mmol). Then sodium tungstate (3.3 g; 10 mmol), 0.5 mL of 4M phosphoric acid were added. The system continued to stir for 1 h under reflux. After the reaction was completed, after the system was cooled to room temperature, a saturated solution of sodium bisulfite was added dropwise, followed by stirring for 10 minutes, the solid was removed by filtration, the filtrate was evaporated to dryness, added ice water and extracted with dichloromethane, the extract was washed and dried, and evaporated to dryness to obtain The crude product was then recrystallized from a solvent to obtain 31.96 g of the target product, yield: 95%, and HPLC content greater than 98%.
Embodiment 2
[0026] Preparation of A-carbo-3,5-cleavage-androst-5-one-3,17β-diacid:
[0027] Add 3-carbonyl-4-androstene-17-carboxylic acid (31.64g; Fw: 316.43 ; 100mmol), then add 100mL1,4-dioxane and hydrogen peroxide (30wt%H 2 o 2 , 150mmol). Then sodium tungstate (3.3 g; 10 mmol), 0.5 mL of 4M nitric acid were added. The system continued to stir for 1 h under reflux. After the reaction was completed, after the system was cooled to room temperature, a saturated solution of sodium bisulfite was added dropwise, followed by stirring for 10 minutes, the solid was removed by filtration, the filtrate was evaporated to dryness, added ice water and extracted with dichloromethane, the extract was washed and dried, and evaporated to dryness to obtain The crude product was then recrystallized from a solvent to obtain 31.96 g of the target product, yield: 95%, and HPLC content greater than 98%.
Embodiment 3
[0029] Preparation of A-carbo-3,5-cleavage-androst-5-one-3,17β-diacid:
[0030] Add 3-carbonyl-4-androstene-17-carboxylic acid (31.64g; Fw: 316.43 ; 100mmol), then add 150mL1,2-dichloroethane and hydrogen peroxide (30wt%H 2 o 2 , 200mmol). Then sodium tungstate (3.3 g; 10 mmol), p-toluenesulfonic acid (1.7 g; Fw: 172.20; 10 mmol) were added. The system continued to stir for 1 h under reflux. After the reaction was completed, after the system was cooled to room temperature, a saturated solution of sodium bisulfite was added dropwise, followed by stirring for 10 minutes, the solid was removed by filtration, the filtrate was evaporated to dryness, added ice water and extracted with dichloromethane, the extract was washed and dried, and evaporated to dryness to obtain The crude product was then recrystallized from a solvent to obtain 32.97 g of the target product, yield: 98%, and HPLC content greater than 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com