Preparation method of ferrocenecarboxylic acid
A technology of ferrocene formic acid and acetyl ferrocene, applied in chemical instruments and methods, metallocene, organic chemistry, etc., can solve the problems of many by-products, low total yield, and high requirements for experimental equipment, and achieves reaction consumption. Short, easy-to-control, inexpensive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In the first step, 60 ml of deionized water and 10 grams of sodium hydroxide were added to the reactor to fully stir and dissolve to form a sodium hydroxide solution; the reactor was cooled to 0-5°C in an ice-water bath, and 2 ml of PEG-400 was added under stirring. (polyethylene glycol) and 16.8 grams of iodine-potassium iodide, then add acetylferrocene powder with a total amount of 2.3 grams in four times within 40 minutes, each time adding an equal amount, and then fully stir for 1h;
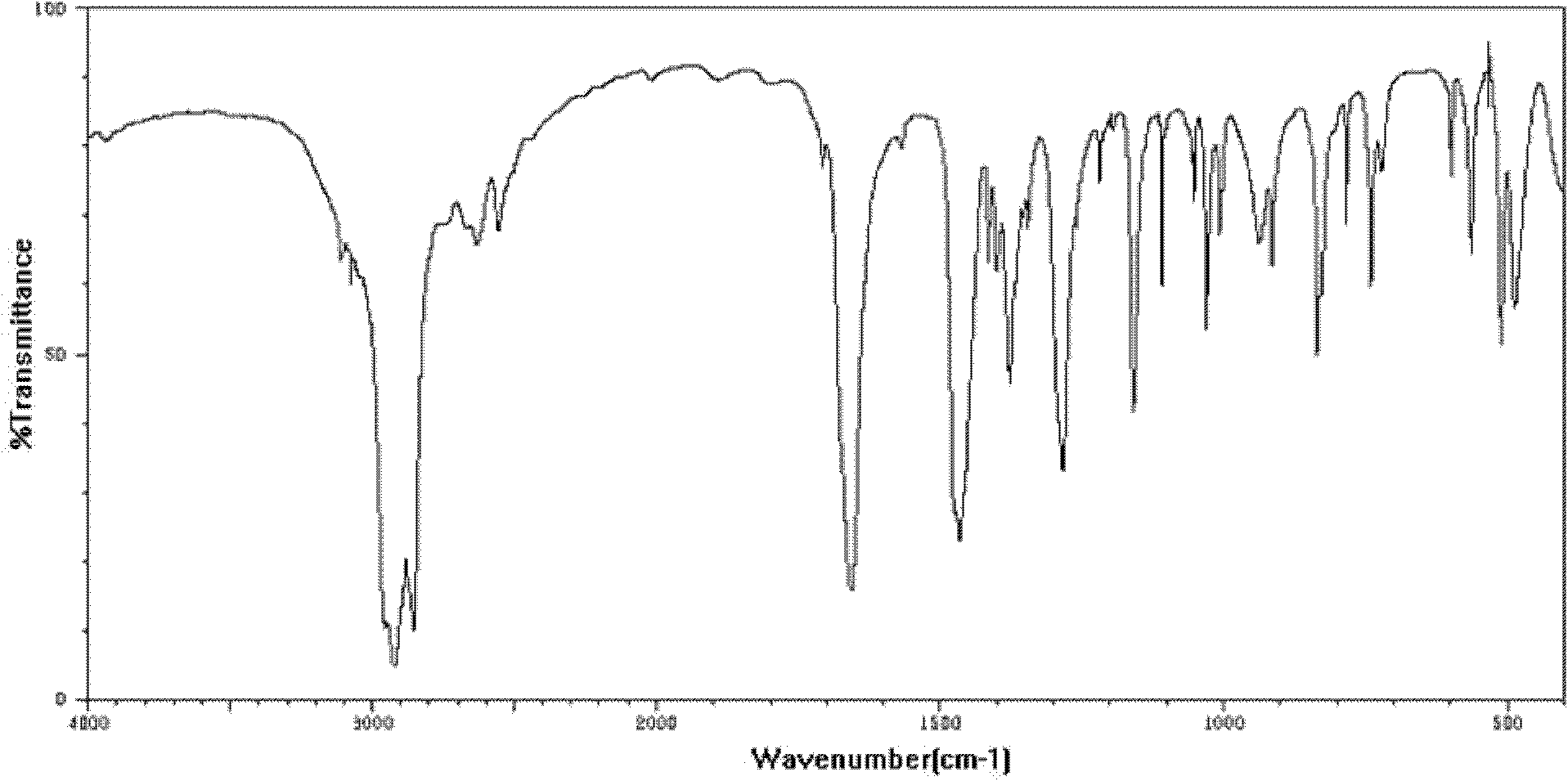
[0023] In the second step, the solution obtained from the first step was extracted and washed twice with dichloromethane, and the aqueous phase was adjusted to pH=2~3 with strong acid, and a yellow precipitate was formed. After cooling, the solution was filtered and washed twice with water. Ferrocenecarboxylic acid, the yield calculated by weighing is 85.6%. see figure 1 As shown, it is the infrared spectrogram of the solid product obtained in Example 1, and it can be seen from the sp...
Embodiment 2
[0025] In the first step, 60 ml of deionized water and 10 g of sodium hydroxide were added to the reactor to fully stir and dissolve to form a sodium hydroxide solution; the reactor was cooled to 10°C with an ice-water bath, and 2 ml of PEG-400 and 16.8 g iodine-potassium iodide, add acetylferrocene powder with a total amount of 2.76 g in batches within 40 minutes, and then fully stir for 1 h;
[0026] In the second step, the solution obtained from the first step was extracted and washed twice with dichloromethane, and the aqueous phase was adjusted to pH=2~3 with strong acid, and a yellow precipitate was formed. After cooling, the solution was filtered and washed twice with water. Ferrocenecarboxylic acid, 84% yield by weight.
Embodiment 3
[0028] In the first step, 60 ml of deionized water and 10 g of sodium hydroxide were added to the reactor to fully stir and dissolve to form a sodium hydroxide solution; the reactor was cooled to 30°C with an ice-water bath, and 2 ml of PEG-400 and 16.8 g iodine-potassium iodide, add acetylferrocene powder with a total amount of 3.22 g in batches within 40 minutes, and then fully stir for 1 h;
[0029] In the second step, the solution obtained from the first step was extracted and washed twice with dichloromethane, and the aqueous phase was adjusted to pH=2~3 with strong acid, and a yellow precipitate was formed. After cooling, the solution was filtered and washed twice with water. Ferrocenecarboxylic acid, the yield calculated by weighing is 79.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com