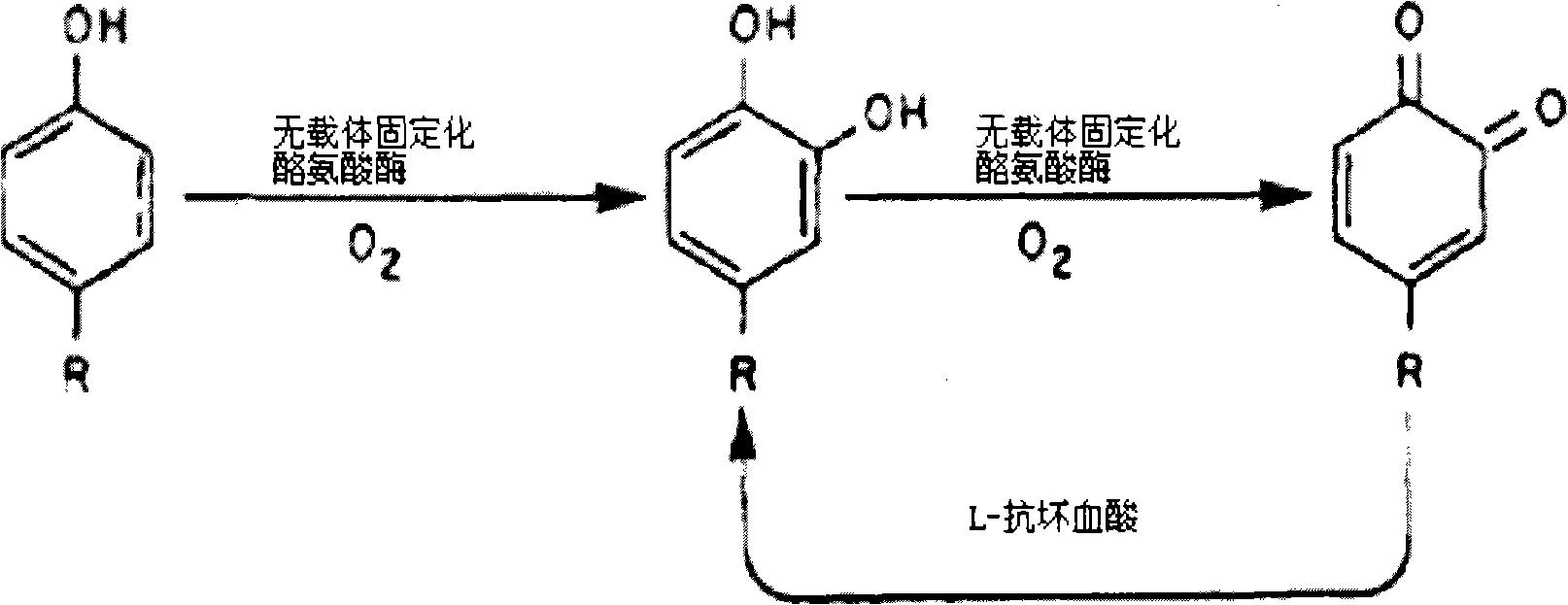
Method for synthesizing medicament L-dopa by enzyme catalysis
A technique for synthesizing the drug, dopa, applied in the field of enzyme-catalyzed synthesis of the drug L-dopa, which achieves the effects of cost reduction, mild reaction conditions, and a single component
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Pack the carrier-free immobilized tyrosinase into a column, let the mixture of L-tyrosine and L-ascorbic acid pass through the column with the carrier-free immobilized tyrosinase, and the effluent product mixture You can get L-dopa. The conversion rate of this method can reach more than 25% after 0.5h.
Embodiment 2
[0039] Add the mixture of carrier-free immobilized tyrosinase, L-tyrosine and L-ascorbic acid into a batch reactor for reaction for a period of time, remove the carrier-free immobilized tyrosinase, and obtain L-dopa . This method reacts for 2 hours, and the conversion rate of more than 60% can be obtained. Carrier-free immobilized tyrosinase can be centrifuged at 6000r / min at room temperature to remove most of it first, and then filter to remove the remaining small part of enzyme powder.
Embodiment 3
[0041]Add carrier-free immobilized tyrosinase into a continuous reactor with a mixture of L-tyrosine and L-ascorbic acid, magnetically stir the reaction, and continuously export the generated product L-dopa, and use the same Introduce the fresh L-tyrosine and L-ascorbic acid reaction solution at a high speed. After 1 hour, the conversion rate can reach more than 50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com