Calf spleen extract for treating tumors
A technology of extract and calf, applied in the direction of anti-tumor drugs, drug combination, drug delivery, etc., can solve the problems of immune function damage, injury, loss of effective treatment opportunities, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
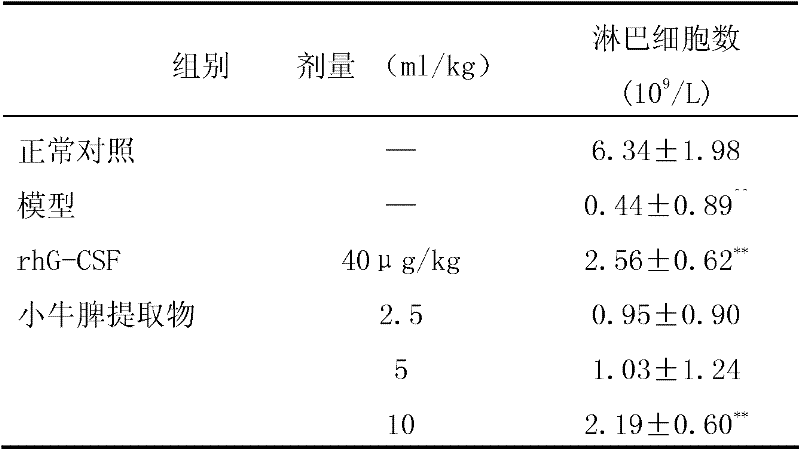
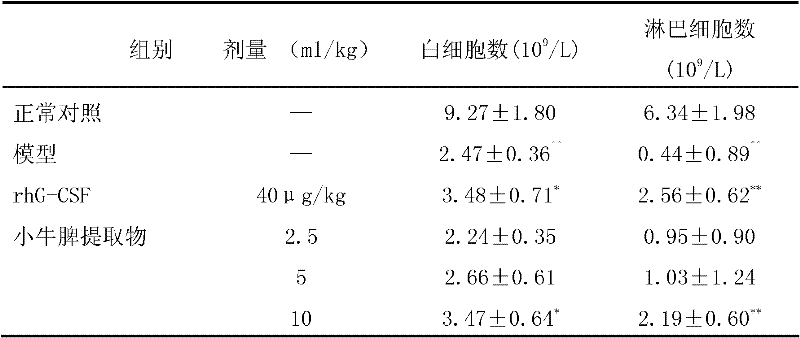
[0164] Get the spleen of dairy cow (within 24 hours after birth), remove connective tissue and fat, wash with 0.9% sodium chloride solution, add 3 times of normal saline to carry out homogenization, generally carry out 3 times, and the colloid mill particle size is 4 μ m; Put the homogenate into the freezer at -20°C for deep freezing. After it is completely frozen, take it out and put it in a water bath below 30°C to thaw. Keep warm for 35 minutes, quickly cool down to 15°C; centrifuge the above solution at a speed of 3500 rpm, temperature below 5°C, centrifuge for 20 minutes, and collect the supernatant; Dalton's membrane ultrafiltration; 0.20μm filter element sterile filtration sub-package to obtain injection, calf spleen extract injection 2.5ml contains 6.25mg polypeptide, 475μg ribose.
Embodiment 2
[0166] Get the spleen of dairy cow (within 24 hours after birth), remove connective tissue and fat, wash with 0.9% sodium chloride solution, add 4 times of normal saline to carry out homogenization, generally carry out 4 times, and the colloidal abrasive particle size is 3 μm; Put the homogenate into the freezer for deep freezing at 30°C. After it is completely frozen, take it out and thaw it in a water bath below 40°C. After it is completely melted, continue to put it into the freezer. Repeat this three times; keep the frozen-thawed liquid at 80°C For 40 minutes, quickly cool down to 20°C; centrifuge the above solution at a speed of 3000 rpm and a temperature below 4°C for 30 minutes, and collect the supernatant; Ultrafiltration with a membrane of Dunton; 0.20μm filter element sterile filtration and repacking to obtain the injection. Calf spleen extract injection 2.5ml contains 9mg polypeptide and 600μg ribose.
Embodiment 3
[0168] Get the spleen of dairy cow (within 24 hours after birth), remove connective tissue and fat, wash with 0.9% sodium chloride solution, add 2 times of normal saline to carry out homogenization, generally carry out 2 times, and the colloid mill particle size is 5 μm; Put the homogenate into the freezer at -15°C for deep freezing. After it is completely frozen, take it out and put it in a water bath below 25°C to thaw. Keep warm for 35 minutes, quickly cool down to 10°C; centrifuge the above solution at a speed of 3000 rpm, temperature below 2°C, and centrifuge for 20 minutes to collect the supernatant; Dalton's membrane ultrafiltration; 0.20μm filter sterile filtration subpackage, that is, the injection. Calf spleen extract injection 2.5ml contains 10.5mg polypeptide and 950μg ribose.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com