Use of 4-alkyl-6-aryl-5-acetyl-1,3-thiazine for preparing neuraminidase inhibitor
A neuraminidase, acetyl group technology, applied in the direction of antiviral agents, active ingredients of heterocyclic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
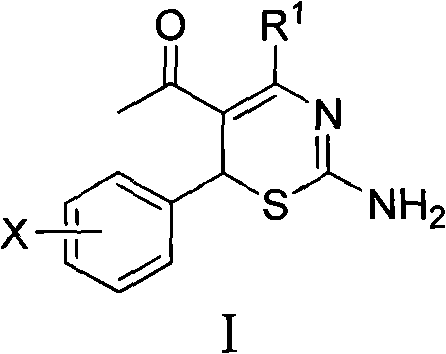
[0019] Preparation of Example 14-Alkyl-6-aryl-5-acetyl-2-amino-1,3-thiazine (I) and its salts
[0020] 4-Alkyl-6-aryl-5-acetyl-2-amino-1,3-thiazine (I) and its salts were prepared according to the literature [CN 201010595032.2].
[0021]
[0022] Wherein, X is selected from: 2-chloro, 2-fluoro, 2-hydroxy, 2-methoxy, 2-ethoxy, 2-nitro, 3-dimethylamino, 3-chloro, 3-bromo, 3 -Fluoro, 3-methyl, 3-ethyl, 3-trifluoromethyl, 3-hydroxy, 3-methoxy, 3-ethoxy, 3-nitro, 3-sulfonic acid, 3-methyl Sulfonylamino, 3-sulfamoyl, 4-dimethylamino, 4-chloro, 4-bromo, 4-fluoro, 4-methyl, 4-ethyl, 4-trifluoromethyl, 4-hydroxyl, 4 -methoxy, 4-ethoxy, 4-acetoxy, 4-nitro, 4-sulfonate, 4-methanesulfonylamino, 4-sulfamoyl, 2-chloro-5-nitro, 3-ethyl-4-hydroxy, 3,4-dimethoxy or 2,4,5-trimethoxy; R 1 Selected from: C 1 ~C 2 Alkyl, C 3 ~C 4 Linear alkyl or branched alkyl; salt selected from hydrochloride, hydrobromide, phosphate, sulfate, nitrate, methanesulfonate or p-toluenesulfonate.
Embodiment 24
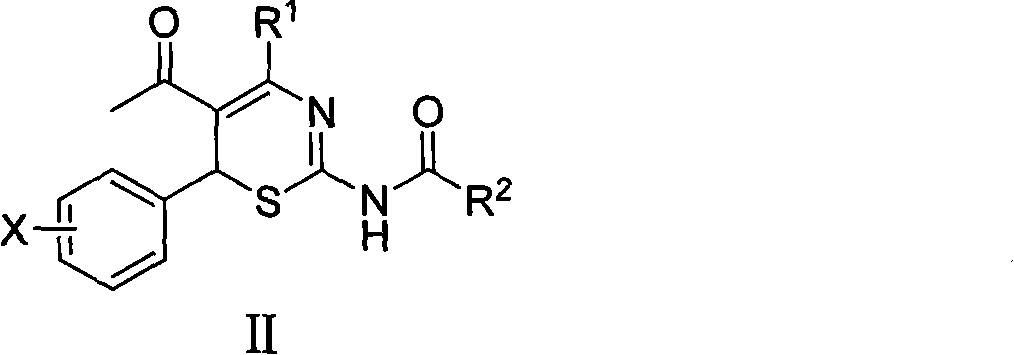
[0023] Preparation of Example 24-Alkyl-6-aryl-5-acetyl-2-acylamino-1,3-thiazine (II) and its salts
[0024] 4-Alkyl-6-aryl-5-acetyl-2-acylamino-1,3-thiazine (II) and its salts were prepared according to the literature [CN 201010595032.2].
[0025]
[0026] Compounds of the invention
[0027] It can be seen from the data in the table that the preferred compounds have better inhibitory activity on neuraminidase and can be applied to the preparation of neuraminidase inhibitors. The inhibitory rate of 4-alkyl-6-aryl-5-acetyl-1,3-thiazine on neuraminidase was higher than that of corresponding 4-alkyl-6-aryl-2-amido-1,3- Thiazine-5-carboxylate has a high rate of inhibition of neuraminidase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com