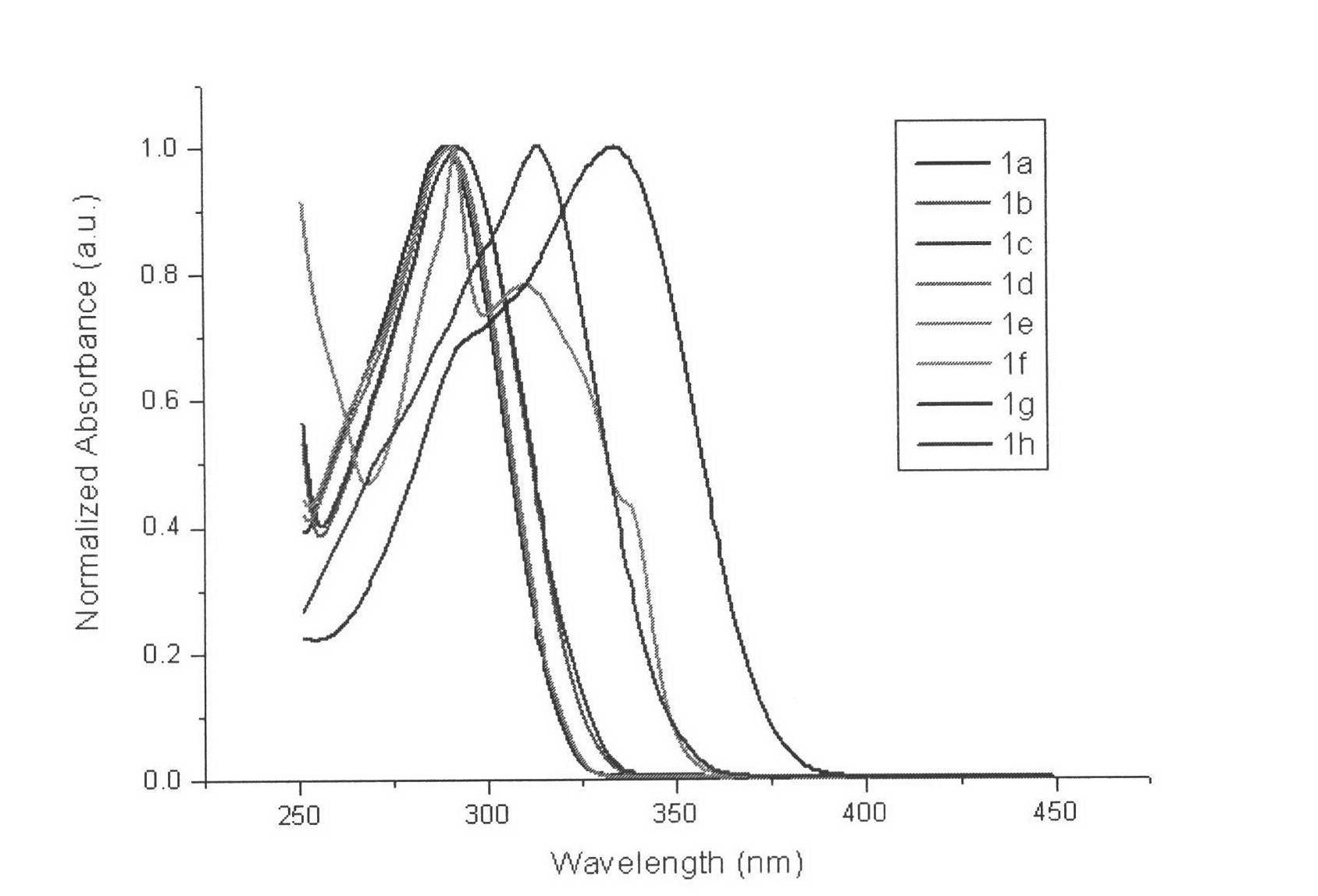
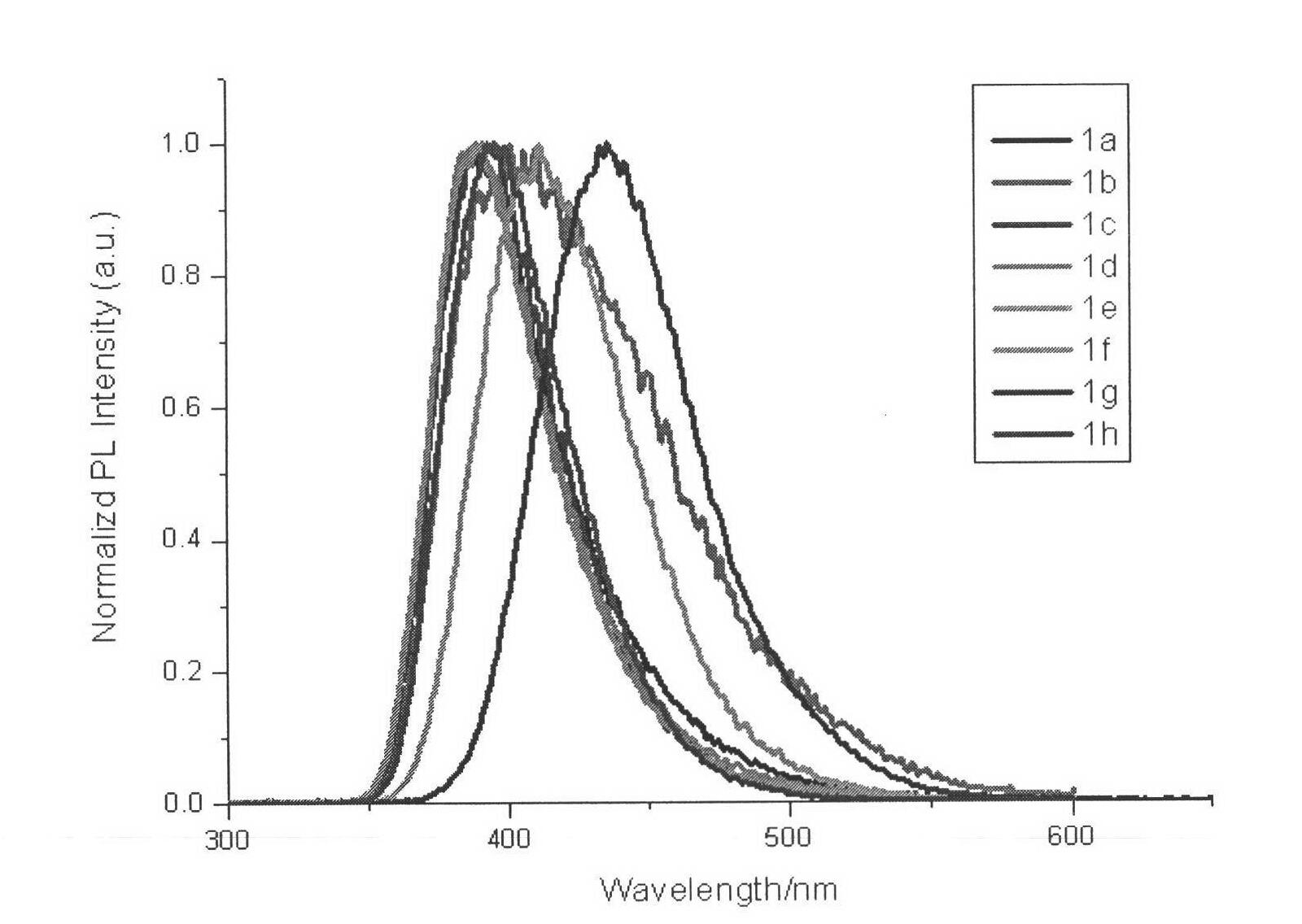
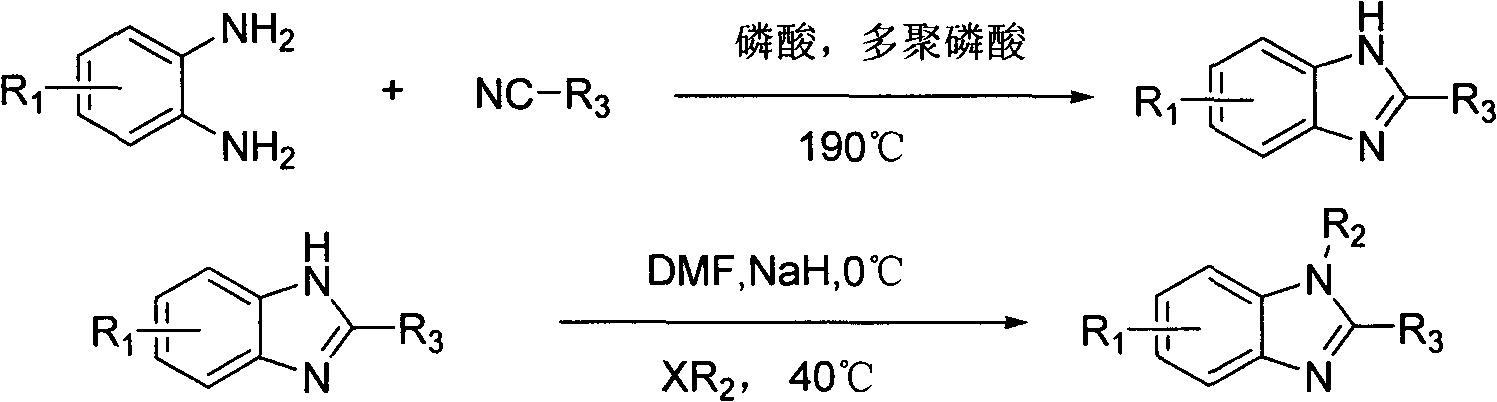
1-alkyl-2-substituted phenyl benzimidazole compound synthesis method and application
A technology of phenylbenzimidazole and synthesis method, which is applied in chemical instruments and methods, organic chemistry, electrical components, etc., and can solve problems such as harsh reaction conditions, high reaction cost, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: 2-(4-phenylenesulfide phenyl)-6-methylbenzimidazole
[0038] Add 2.44g (20mmol) of 4-methyl o-phenylenediamine, 4.22g (20mmol) of p-phenylene sulfide benzonitrile, 20mL of phosphoric acid, and 25mL of polyphosphoric acid into a 50mL four-necked flask, and slowly heat up to 190°C. React at this temperature for 12 hours, cool to 70°C, pour the reaction solution into 500mL of water, precipitate a yellow-brown solid, filter with suction, adjust the pH of the filter cake to 8 with 10% sodium hydroxide solution, heat to reflux for 8 hours, and heat filter to obtain the crude product. Then recrystallized from absolute ethanol to obtain 5.19 g of light yellow solid with a yield of 82.2%.
Embodiment 2
[0039] Example 2: Synthesis of 2-(4-(p-tert-butylphenylsulfide) phenyl)-6-methylbenzimidazole
[0040] Add 2.44g (20mmol) of 4-methyl-o-phenylenediamine, 5.34g (20mmol) of 4-(p-tert-butylphenylsulfide)benzonitrile, 20mL of phosphoric acid, and 25mL of polyphosphoric acid into a 50mL four-necked flask. Slowly raise the temperature to 190°C, react at this temperature for 12 hours, cool to 70°C, pour the reaction solution into 500mL of water, a yellow solid precipitates, filter with suction, adjust the pH of the filter cake to 8 with 10% sodium hydroxide solution, and heat to reflux for 8h , hot filtration to obtain the crude product, and then recrystallized from absolute ethanol to obtain 5.96 g of off-white solid, with a yield of 80.2%.
Embodiment 3
[0041] Embodiment 3: the synthesis of 2-(4-phenoxyphenyl)-6-methylbenzimidazole
[0042] Add 2.44g (20mmol) of 4-methyl o-phenylenediamine, 4.00g (20mmol) of p-phenoxybenzonitrile, 20mL of phosphoric acid, and 25mL of polyphosphoric acid into a 50mL four-neck flask, and slowly heat up to 190°C. React at high temperature for 12 hours, cool to 70°C, pour the reaction solution into 500mL water, precipitate a yellow solid, filter with suction, adjust the pH of the filter cake to 8 with 10% sodium hydroxide solution, heat and reflux for 8 hours, heat filter to obtain the crude product, and then pass Recrystallization from absolute ethanol gave 5.00 g of off-white solid with a yield of 83.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com